The reason for PTFE's remarkable chemical resistance lies in its unique molecular structure. Polytetrafluoroethylene (PTFE) is built on an extremely strong and stable bond between carbon and fluorine atoms, creating a material that is virtually impervious to attack from nearly all common solvents, acids, and bases.
PTFE’s chemical inertness is not a superficial quality; it is a fundamental property derived from its molecular architecture. The powerful carbon-fluorine bonds form a protective chemical shield, making it one of the most non-reactive plastics known.

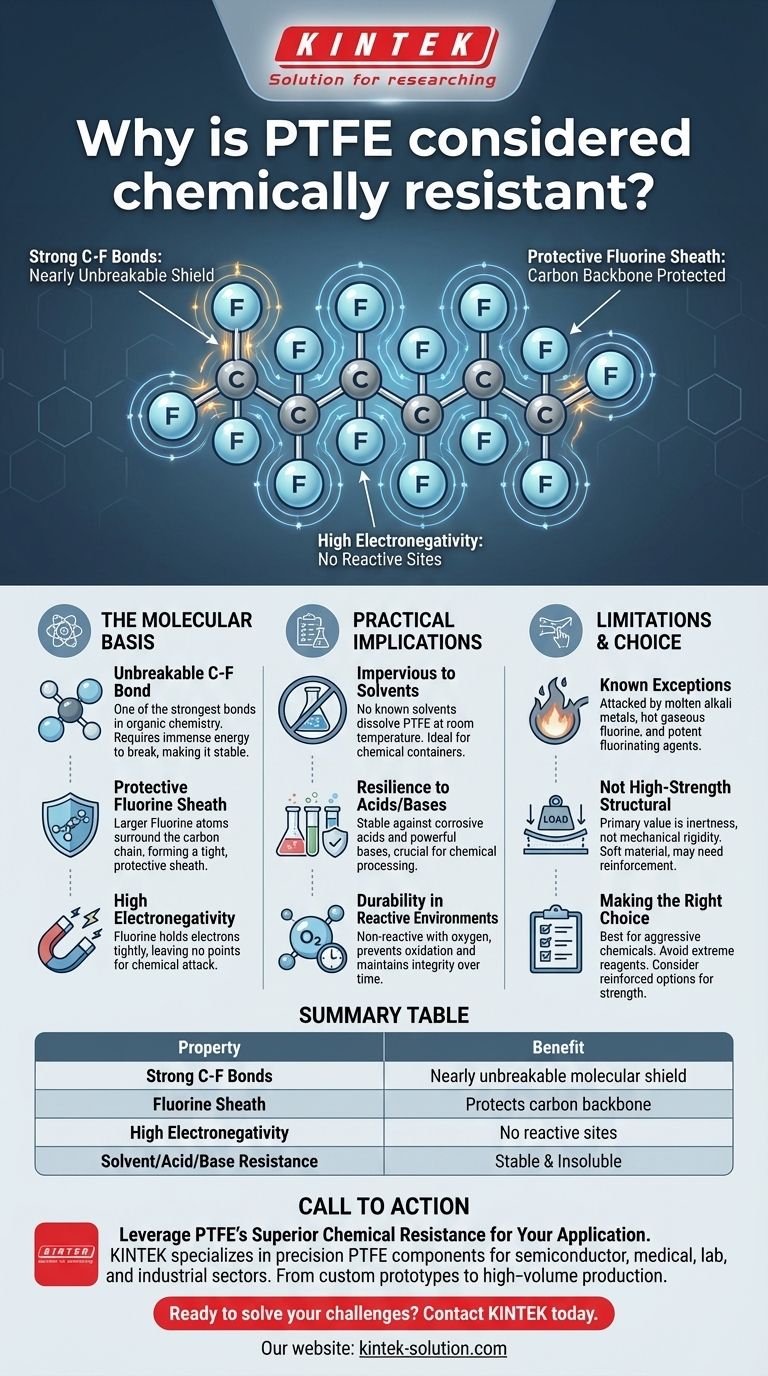
The Molecular Basis of PTFE's Inertness
To truly understand why PTFE is so durable, we must look at its chemical composition. The material's properties are a direct result of the interactions between its atoms.
The Unbreakable Carbon-Fluorine Bond
The bond between a carbon atom and a fluorine atom is one of the strongest single bonds in organic chemistry. This immense strength means a significant amount of energy is required to break it, which most chemicals simply cannot provide.
A Protective Fluorine Sheath
In the PTFE polymer chain, the carbon backbone is completely surrounded by fluorine atoms. These fluorine atoms are larger than the carbon atoms and effectively create a tight, uniform "sheath" that shields the more vulnerable carbon chain from outside chemical attacks.
High Electronegativity
Fluorine is the most electronegative element. This means it holds onto its electrons very tightly, leaving no easy points of attack for other reactive molecules to latch onto. This lack of available electrons makes the surface of PTFE extremely non-reactive.
Practical Implications of Extreme Resistance
This molecular stability translates directly into reliable performance in the most demanding environments.
Impervious to Solvents
PTFE is famously non-soluble. There are no known solvents that can dissolve it at or near room temperature, making it ideal for containers, seals, and linings that come into contact with a wide variety of chemicals.
Resilience Against Acids and Bases
From highly corrosive acids to powerful bases, PTFE remains stable and does not degrade. This makes it an essential material in chemical processing, manufacturing, and laboratory settings where equipment is constantly exposed to aggressive substances.
Durability in Reactive Environments
The material does not react with common reactive substances, including oxygen. This prevents oxidation and ensures the material maintains its integrity over long periods, even when exposed to harsh conditions.
Understanding the Limitations and Trade-offs
While PTFE is exceptionally resistant, it is not completely invincible. Understanding its few weaknesses is critical for proper application.
The Few Known Exceptions
Only a handful of extremely aggressive substances can affect PTFE. These include molten alkali metals (like sodium), hot gaseous fluorine, and potent fluorinating agents like chlorine trifluoride and oxygen difluoride.
Not a High-Strength Structural Material
PTFE's primary strength is its chemical inertness, not its mechanical rigidity. It is a relatively soft material, which means it may not be suitable for high-load structural components without reinforcement. Its value is in its surface properties and stability, not its raw strength.
Making the Right Choice for Your Application
Leveraging PTFE effectively means matching its unique properties to your specific operational needs.
- If your primary focus is handling aggressive acids, bases, or organic solvents: PTFE is the industry standard and one of the most reliable choices for preventing material degradation and contamination.
- If your environment involves extreme reagents like molten alkali metals or elemental fluorine: You must seek alternative materials, as these are the few known substances capable of chemically attacking PTFE.
- If you need both chemical resistance and high mechanical strength: Consider reinforced PTFE options or composite materials that combine PTFE's inert surface with a stronger structural core.
Ultimately, understanding the molecular strength of PTFE allows you to confidently leverage its near-universal chemical resistance in the most demanding applications.
Summary Table:
| Property | Benefit |
|---|---|
| Strong C-F Bonds | Creates a nearly unbreakable molecular shield |
| Fluorine Sheath | Protects the carbon backbone from chemical attack |
| High Electronegativity | Leaves no reactive sites for other molecules |
| Solvent Resistance | Insoluble in virtually all common solvents |
| Acid/Base Resistance | Stable even with highly corrosive substances |
Leverage PTFE's Superior Chemical Resistance for Your Application
PTFE's unmatched inertness makes it the ideal material for critical components in harsh environments. Whether you need seals, liners, or custom labware that can withstand aggressive chemicals without degrading, KINTEK has the expertise.
We specialize in manufacturing precision PTFE components for the semiconductor, medical, laboratory, and industrial sectors. From custom prototypes to high-volume production, we ensure your parts meet the highest standards of chemical resistance and performance.
Ready to solve your chemical resistance challenges? Contact KINTEK today to discuss your specific needs and get a quote for reliable PTFE solutions.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Seals Filter Holders for Versatile Applications
People Also Ask
- How was Teflon discovered and when was it commercialized? The Accidental Invention of a Wonder Polymer
- Why is PTFE compliance with USDA and FDA standards important? Ensure Safety in Food, Pharma & Medical
- What is the chemical composition of Teflon? The Science Behind Its Non-Stick Properties
- What are the main applications of PTFE material? Unlock Superior Performance in Demanding Industries
- What are the main properties of Teflon material? Unrivaled Chemical Resistance & Non-Stick Performance
- What are the key mechanical properties of Teflon? Leveraging Low Friction & Chemical Inertness
- What are the limitations of PTFE materials? Understand the Key Trade-Offs Before You Spec
- What are the mechanical properties of PTFE? Leverage Its Low Friction & Chemical Resistance



















