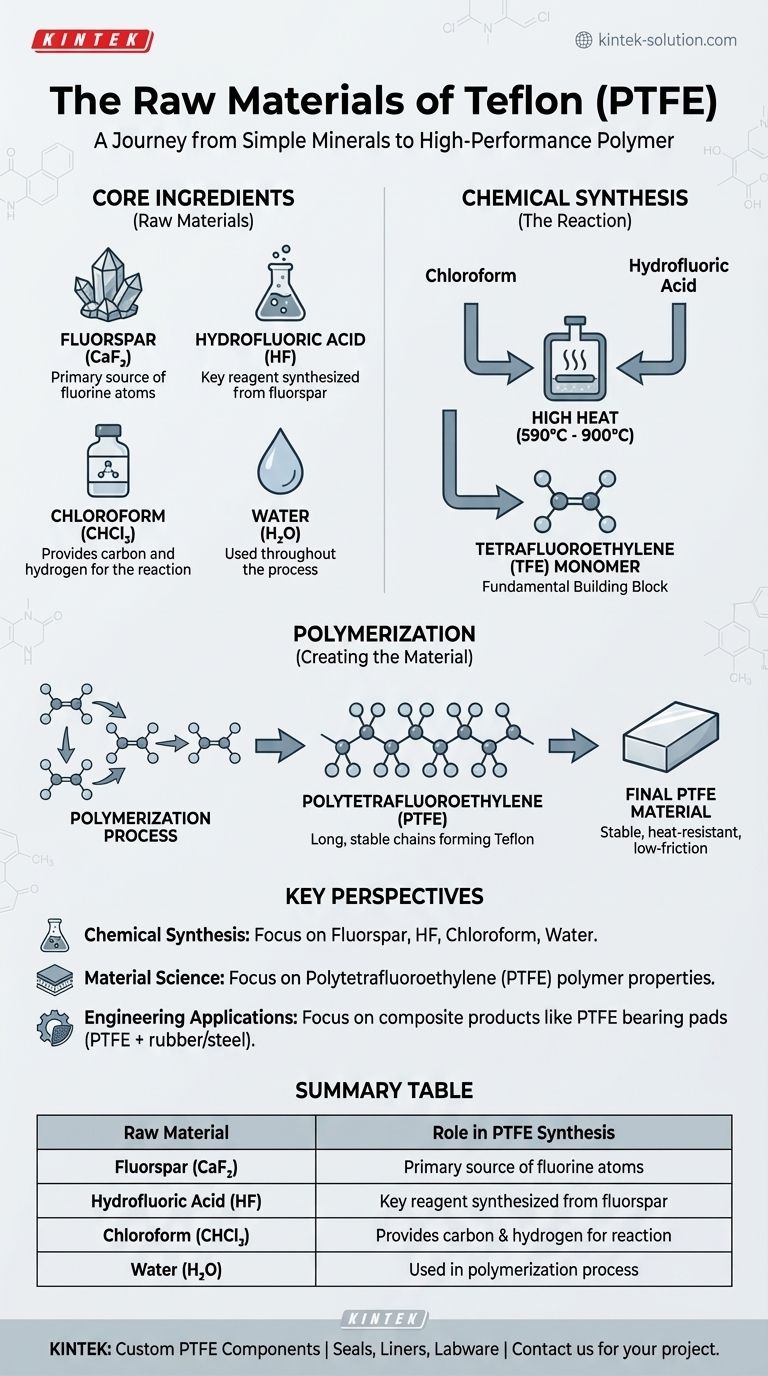
In short, Teflon is created from four key raw materials: fluorspar, hydrofluoric acid, chloroform, and water. These ingredients undergo a chemical synthesis process under high heat to produce the final material.
While the raw ingredients are simple minerals and chemicals, the manufacturing process transforms them into Polytetrafluoroethylene (PTFE)—a highly stable and uniquely non-reactive polymer that we know as Teflon.

From Minerals to Polymer: The Teflon Synthesis Process
Understanding Teflon begins with understanding how its raw materials are transformed. The process is a multi-step chemical synthesis designed to create a very specific molecular structure.
The Core Ingredients
- Fluorspar: This is a mineral form of calcium fluoride (CaF₂). It is the primary source of the fluorine atoms that give Teflon its unique properties.
- Hydrofluoric Acid (HF): This highly corrosive acid is synthesized from fluorspar and serves as a key reagent in the process.
- Chloroform (CHCl₃): A common industrial solvent, chloroform provides the carbon and hydrogen atoms needed for the reaction.
- Water (H₂O): Water is used throughout the manufacturing process, often as part of the polymerization stage.
The Chemical Reaction
The core of the manufacturing process involves reacting chloroform and hydrofluoric acid. This reaction produces an intermediate gas called tetrafluoroethylene (TFE). This is the fundamental building block, or monomer, of Teflon.
This initial synthesis occurs in a chemical reaction chamber heated to extremely high temperatures, typically between 590°C and 900°C (1094°F to 1652°F).
Creating the Final Material: Polymerization
Once the TFE gas is created, it undergoes a process called polymerization.
During polymerization, individual TFE molecules are linked together to form long, stable chains. The result is Polytetrafluoroethylene (PTFE), the solid polymer we recognize as Teflon. This final material is exceptionally stable and resistant to heat, chemicals, and friction.
Understanding the Trade-offs and Misconceptions
While the end product is inert and safe for most applications, the manufacturing process and its history involve complexities worth noting.
Confusion with Composite Products
It's important to distinguish between raw Teflon and products that incorporate it. For instance, Teflon bearing pads are composite materials.
These pads are made by bonding a sheet of PTFE onto a base of natural or chloroprene rubber, often reinforced with steel. In this case, rubber and steel are not raw materials of Teflon, but components of the final product.
Focus on Material Properties
The value of Teflon comes from the properties created during synthesis. The strong carbon-fluorine bonds make PTFE resistant to nearly all chemicals, give it a very high melting point, and create an extremely low-friction surface.
These properties are a direct result of its molecular structure, not the initial raw materials themselves. The manufacturing process is what unlocks this performance.
Applying This to Your Understanding
The key is to differentiate between the chemical composition of Teflon itself and the components of products that use it.
- If your primary focus is on chemical synthesis: The core raw materials are fluorspar, hydrofluoric acid, chloroform, and water, which are used to create the TFE monomer.
- If your primary focus is on material science: The essential substance is Polytetrafluoroethylene (PTFE), a polymer formed by linking TFE molecules into long, stable chains.
- If your primary focus is on engineering applications: You will encounter composite products where PTFE is bonded to other materials like rubber or steel to achieve specific mechanical goals.
Ultimately, the journey from simple minerals to a high-performance polymer is a testament to the power of precise chemical engineering.
Summary Table:
| Raw Material | Role in PTFE Synthesis |
|---|---|
| Fluorspar (CaF₂) | Primary source of fluorine atoms |
| Hydrofluoric Acid (HF) | Key reagent synthesized from fluorspar |
| Chloroform (CHCl₃) | Provides carbon and hydrogen for the reaction |
| Water (H₂O) | Used in the polymerization process |
Need High-Quality, Custom PTFE Components?
KINTEK specializes in the precision manufacturing of PTFE components—from seals and liners to custom labware. Our expertise in material science ensures you get parts that leverage the full chemical resistance and low-friction properties of Teflon for your most demanding applications in the semiconductor, medical, laboratory, and industrial sectors.
Whether you need prototypes or high-volume orders, we provide custom fabrication to meet your exact specifications. Contact KINTEK today to discuss your project and get a quote!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What are the key advantages of PTFE? Unmatched Performance for Extreme Environments
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs



















