At its core, Polytetrafluoroethylene (PTFE) is chemically resistant because of the exceptionally strong bonds between its carbon and fluorine atoms. This molecular stability creates a non-reactive surface, making PTFE an invaluable material for applications where it must withstand aggressive acids, bases, and solvents without degrading.
The true advantage of PTFE lies in its molecular armor. The powerful carbon-fluorine bond makes it nearly inert, which translates directly into superior reliability, safety, and longevity for components used in harsh chemical environments.

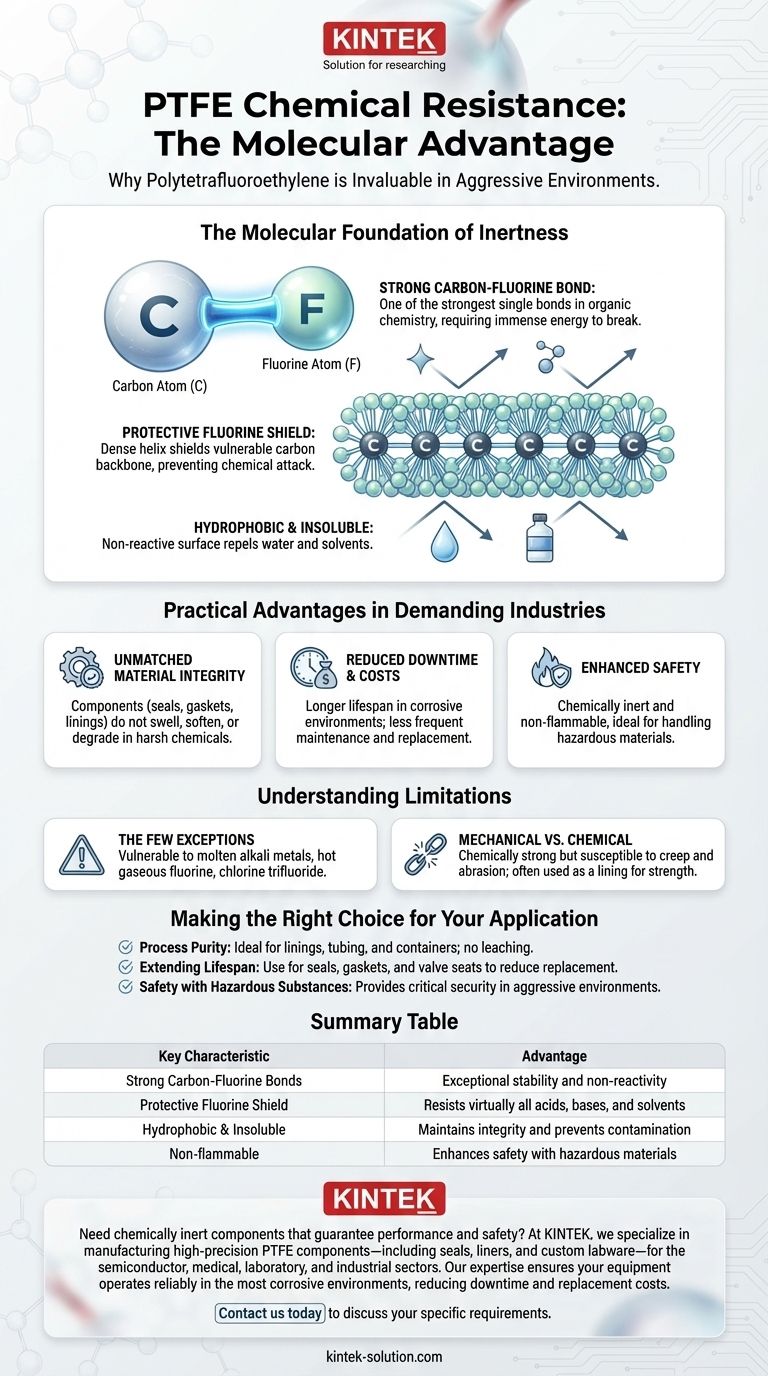
The Molecular Foundation of PTFE's Inertness
To understand why PTFE is so robust, we must look at its chemical structure. Its properties are not accidental; they are a direct result of its atomic makeup.
The Unbreakable Carbon-Fluorine Bond
The bond between carbon and fluorine atoms is one of the strongest single bonds in organic chemistry. This immense strength means a tremendous amount of energy is required to break it, making the molecule highly stable and non-reactive.
A Protective Fluorine Shield
In the PTFE molecule, a chain of carbon atoms forms a backbone, which is completely sheathed by a dense helix of fluorine atoms. This outer layer of fluorine effectively shields the vulnerable carbon backbone from any potential chemical attack.
Consequence: Chemical Indifference
This combination of strong bonds and a protective shield renders PTFE hydrophobic (water-repelling) and insoluble in virtually all solvents at room temperature. It simply does not offer a reaction site for other chemicals to latch onto.
Practical Advantages in Demanding Industries
This inherent chemical resistance provides tangible benefits that are critical for performance, safety, and cost-effectiveness in many sectors.
Unmatched Material Integrity
Components made from PTFE, such as seals, gaskets, linings, and valves, do not swell, soften, or degrade when exposed to the vast majority of industrial chemicals. This ensures that their physical and mechanical properties remain consistent over time.
Reduced Downtime and Replacement Costs
Because PTFE components last longer in corrosive environments, equipment requires less frequent maintenance and replacement. This directly reduces material costs and, more importantly, minimizes costly operational downtime.
Enhanced Safety with Hazardous Materials
PTFE is not only chemically inert but also non-flammable. This makes it an ideal choice for handling aggressive and volatile substances where a material failure could have catastrophic consequences.
Understanding the Limitations
While its chemical resistance is exceptional, PTFE is not universally invincible. An objective assessment requires acknowledging its specific, albeit rare, vulnerabilities.
The Few Chemical Exceptions
Only a handful of extremely reactive substances can attack PTFE. These include molten alkali metals (like sodium), hot gaseous fluorine, and potent agents like chlorine trifluoride. For all standard acids, bases, and solvents, it remains inert.
Mechanical vs. Chemical Strength
PTFE's primary strength is chemical, not mechanical. It is a relatively soft material susceptible to creep and abrasion. In applications requiring high structural integrity, it is often used as a lining or coating for a stronger base material like steel.
Making the Right Choice for Your Application
Selecting PTFE should be a decision based on its unique strengths relative to your specific operational goal.
- If your primary focus is process purity: PTFE is an ideal choice for linings, tubing, and containers, as it will not react with or leach into the chemicals it contains.
- If your primary focus is extending equipment lifespan: Using PTFE for seals, gaskets, and valve seats in corrosive environments directly reduces replacement frequency and maintenance costs.
- If your primary focus is safety with hazardous substances: PTFE's chemical inertness provides a critical layer of security when handling the most aggressive industrial chemicals.
Ultimately, understanding the molecular basis of PTFE's resistance allows you to deploy it with confidence where it matters most.
Summary Table:
| Key Characteristic | Advantage |
|---|---|
| Strong Carbon-Fluorine Bonds | Exceptional stability and non-reactivity |
| Protective Fluorine Shield | Resists virtually all acids, bases, and solvents |
| Hydrophobic & Insoluble | Maintains integrity and prevents contamination |
| Non-flammable | Enhances safety with hazardous materials |
Need chemically inert components that guarantee performance and safety?
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment operates reliably in the most corrosive environments, reducing downtime and replacement costs.
Contact us today to discuss your specific requirements, from prototypes to high-volume orders.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Customizable PTFE Seals Filter Holders for Versatile Applications
People Also Ask
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- In which industries is PTFE commonly used? Key Applications for Chemical & Thermal Resistance
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments



















