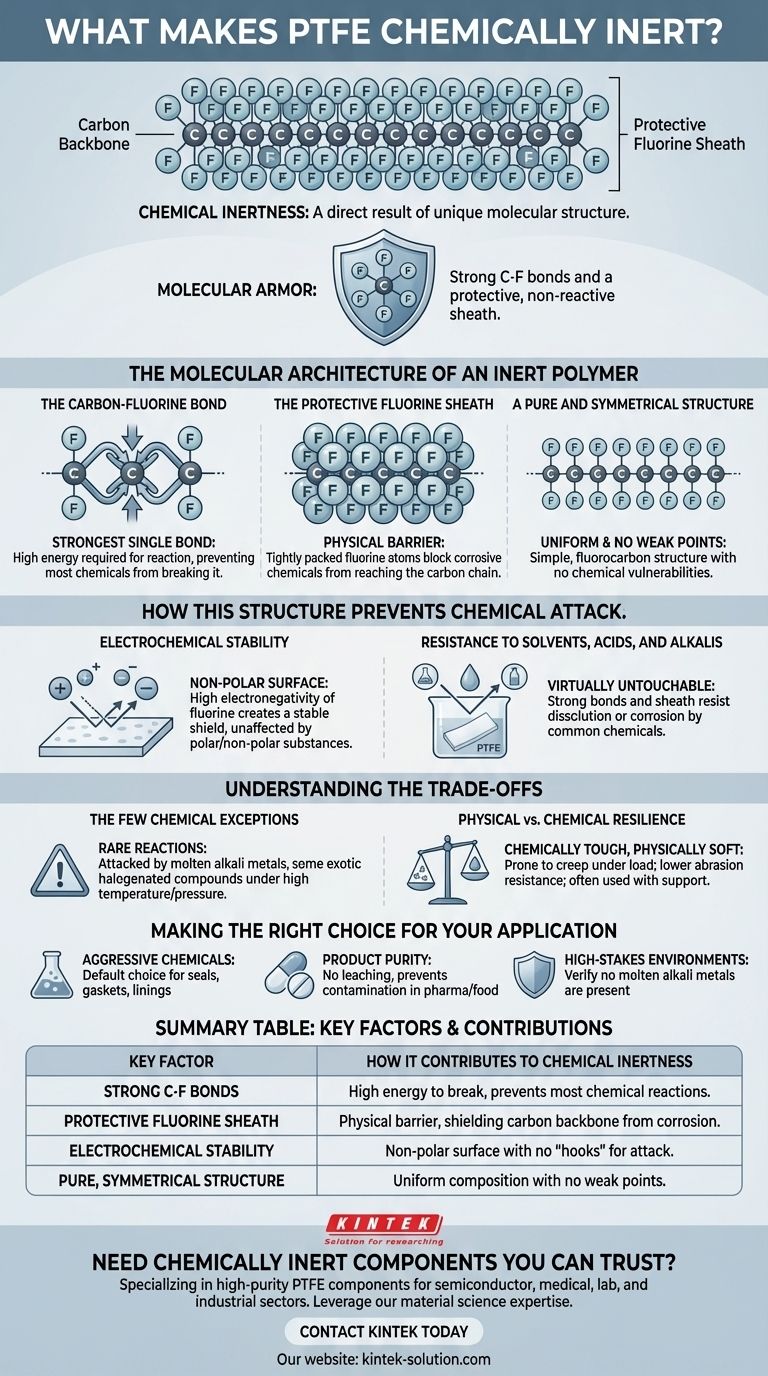
The chemical inertness of Polytetrafluoroethylene (PTFE) is a direct result of its unique molecular structure. Its strength comes from the incredibly powerful carbon-fluorine bonds and the way the fluorine atoms form a protective, non-reactive sheath around the carbon backbone. This combination makes the molecule exceptionally stable and resistant to attack from almost all chemicals.
At its core, PTFE's near-total chemical resistance isn't magic; it's a function of molecular armor. The fluorine atoms create a tightly packed, electrochemically stable shield that physically and chemically blocks other substances from reacting with the vulnerable carbon chain at its center.

The Molecular Architecture of an Inert Polymer
To understand PTFE's resilience, we must look at its atomic-level construction. The properties that make it so useful in chemical plants, laboratories, and pharmaceutical manufacturing are not accidental; they are fundamental to its design.
The Carbon-Fluorine Bond
The bond between a carbon atom and a fluorine atom is one of the strongest single bonds known in organic chemistry. It requires a tremendous amount of energy to break this bond, which is a prerequisite for any chemical reaction to occur. Since most chemicals cannot muster the energy to break it, reactions simply don't start.
The Protective Fluorine Sheath
Fluorine atoms are larger than the hydrogen atoms they replace in a typical polyethylene structure. These larger atoms pack tightly together, forming a continuous, helical sheath around the polymer's carbon backbone. This sheath acts as a physical barrier, preventing corrosive chemicals from even reaching the central carbon chain.
A Pure and Symmetrical Structure
PTFE is a fluorocarbon, meaning it is composed purely of carbon and fluorine. This uniformity means there are no weak points or different types of bonds along the chain that a chemical could target. Its simple, repetitive structure contributes directly to its consistent and predictable inertness.
How This Structure Prevents Chemical Attack
The molecular architecture translates directly into real-world performance. The fluorine sheath doesn't just block chemicals; it actively repels them, making the surface uniquely non-receptive to interaction.
Electrochemical Stability
Fluorine is the most electronegative element. This means it pulls the bonding electrons very close to itself, creating a very stable, non-polar molecule. This lack of polarity makes PTFE unaffected by nearly all polar and non-polar solvents, acids, and bases, as there is no electrochemical "hook" for them to attach to.
Resistance to Solvents, Acids, and Alkalis
Because of the strong bonds and protective sheath, virtually no common substance can dissolve or corrode PTFE. It remains stable when exposed to highly aggressive acids, powerful alkalis, and a vast range of organic solvents, which would quickly degrade most other materials.
Understanding the Trade-offs
While PTFE is exceptionally inert, no material is perfect. Acknowledging its limitations is critical for proper application in demanding environments.
The Few Chemical Exceptions
PTFE's inertness is not absolute. It can be attacked by a very small number of highly reactive substances under specific conditions. These include molten alkali metals (like sodium), and some exotic halogenated compounds like chlorine trifluoride, particularly at high temperatures and pressures.
Physical vs. Chemical Resilience
It is crucial to distinguish chemical inertness from physical properties. While chemically tough, PTFE is a relatively soft material. It can be prone to creep (cold flow) under sustained load and has lower abrasion resistance than many engineering plastics. This is why it is often used in blends or with structural support, like the metal springs in PTFE seals.
Making the Right Choice for Your Application
Understanding the why behind PTFE's inertness allows for confident and precise material selection for your specific industrial challenge.
- If your primary focus is handling aggressive chemicals (acids, bases, solvents): PTFE's stable carbon-fluorine bond structure makes it the default choice for critical components like gaskets, seals, and tank linings.
- If your primary focus is maintaining product purity (pharmaceuticals, food processing): The non-reactive fluorine sheath ensures no chemicals will leach from the material, preventing contamination of sensitive products.
- If your primary focus is a high-stakes environment: Always verify that your process conditions do not involve the few specific chemicals, such as molten alkali metals, known to react with PTFE.
Ultimately, trusting PTFE in your application comes from understanding that its resilience is built into its very molecular fabric.
Summary Table:
| Key Factor | How it Contributes to Chemical Inertness |
|---|---|
| Strong C-F Bonds | The carbon-fluorine bond is extremely strong, requiring high energy to break, which prevents most chemical reactions. |
| Protective Fluorine Sheath | A tight layer of fluorine atoms acts as a physical barrier, shielding the carbon backbone from corrosive substances. |
| Electrochemical Stability | High electronegativity of fluorine creates a non-polar surface with no "hooks" for other chemicals to attack. |
| Pure, Symmetrical Structure | A uniform composition of only carbon and fluorine provides no weak points for chemical degradation. |
Need Chemically Inert Components You Can Trust?
At KINTEK, we specialize in manufacturing high-purity PTFE components—including seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures that the inherent chemical resistance of PTFE is precision-engineered into every part, from custom prototypes to high-volume production runs.
Leverage our material science expertise to protect your critical processes from corrosive chemicals and ensure product purity.
Contact KINTEK today to discuss your application requirements and receive a quote for reliable PTFE solutions.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Seals Filter Holders for Versatile Applications
People Also Ask
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables



















