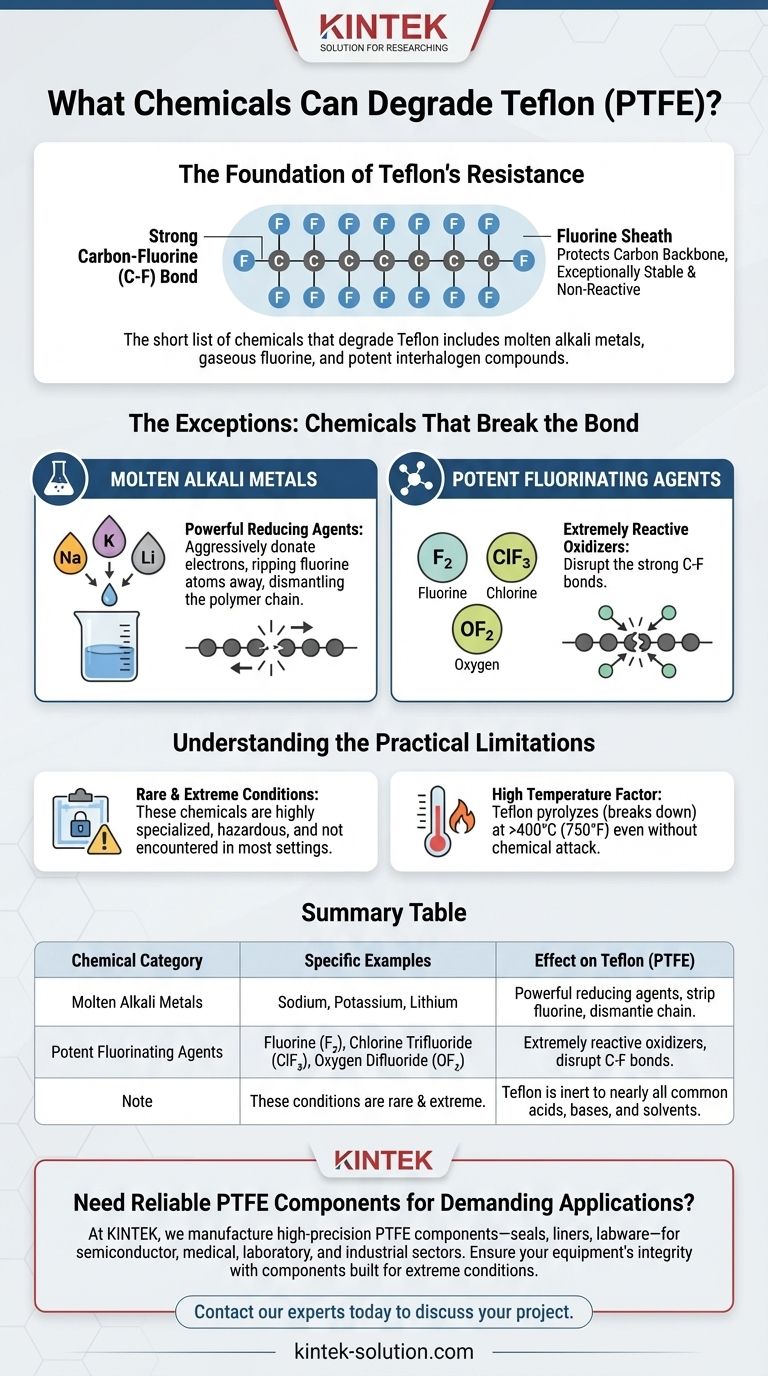
The short list of chemicals that degrade Teflon includes molten alkali metals, gaseous fluorine, and potent interhalogen compounds like chlorine trifluoride and oxygen difluoride. These substances are exceptionally reactive and represent the few known exceptions to Teflon's legendary chemical inertness.
While Teflon (PTFE) is renowned for being one of the most chemically resistant materials available, its stability is not absolute. Its near-invincibility is overcome only by a small, specific group of extremely aggressive reagents that can break its powerful carbon-fluorine bonds.

The Foundation of Teflon's Resistance
The Carbon-Fluorine Bond
The extraordinary resilience of Teflon, technically known as Polytetrafluoroethylene (PTFE), comes from its molecular structure. It is composed of a long chain of carbon atoms, where each carbon is bonded to two fluorine atoms.
The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry.
A Protective Fluorine Sheath
Furthermore, the fluorine atoms are larger than the carbon atoms they are attached to. They effectively form a tight, protective "sheath" around the carbon backbone.
This sheath physically shields the carbon chain from potential chemical attacks, making the entire molecule exceptionally stable and non-reactive.
The Exceptions: Chemicals That Break the Bond
Only a few substances are reactive enough to overcome this formidable chemical defense. They fall into two main categories.
Molten Alkali Metals
Chemicals like molten sodium, potassium, and lithium can degrade Teflon. These metals are powerful reducing agents, meaning they aggressively donate electrons.
In this high-energy state, they can rip fluorine atoms away from the carbon backbone, effectively dismantling the polymer chain and destroying the material.
Potent Fluorinating Agents
A few gaseous compounds can attack Teflon, essentially fighting fire with more fire. These include elemental fluorine (F₂), chlorine trifluoride (ClF₃), and oxygen difluoride (OF₂).
These are some of the most powerful oxidizing and fluorinating agents known. Their extreme reactivity allows them to disrupt the stable C-F bonds that constitute the Teflon polymer.
Understanding the Practical Limitations
These Conditions are Extremely Rare
It is critical to understand that the chemicals capable of degrading Teflon are highly specialized, hazardous, and not encountered in the vast majority of industrial, commercial, or laboratory settings.
For nearly all common acids, bases, solvents, and oxidizers, Teflon remains completely inert.
High Temperature is a Key Factor
Like all polymers, Teflon has a temperature limit. While it has excellent thermal stability, it will begin to break down (pyrolyze) at very high temperatures (above 400°C or 750°F), even without chemical attack.
This thermal degradation is a separate failure mode from a direct chemical assault and is a more common limiting factor in practical applications.
Making the Right Choice for Your Application
Understanding these limits ensures you are using the material correctly and safely.
- If your primary focus is general chemical processing: Teflon is almost certainly an excellent choice, as it resists virtually all common acids, solvents, and bases.
- If you work with high-energy or niche chemistry: For applications involving molten alkali metals (e.g., certain reactors) or aggressive fluorinating agents (e.g., rocket propellants, semiconductor manufacturing), you must seek an alternative material.
- If your primary focus is high-temperature environments: You must pay close attention to Teflon's specified service temperature limits, as thermal degradation is a more likely point of failure than chemical attack.
Ultimately, Teflon's reputation is well-earned, and its limitations only surface under the most extreme and specific chemical conditions.
Summary Table:
| Chemical Category | Specific Examples | Effect on Teflon (PTFE) |
|---|---|---|
| Molten Alkali Metals | Sodium, Potassium, Lithium | Powerful reducing agents that strip fluorine atoms, dismantling the polymer chain. |
| Potent Fluorinating Agents | Fluorine (F₂), Chlorine Trifluoride (ClF₃), Oxygen Difluoride (OF₂) | Extremely reactive oxidizers that disrupt the strong carbon-fluorine bonds. |
| Note | These conditions are rare and extreme. Teflon is inert to nearly all common acids, bases, and solvents. |
Need Reliable PTFE Components for Demanding Applications?
While Teflon's chemical resistance is legendary, selecting the right grade and fabrication method is critical for performance in specialized environments.
At KINTEK, we manufacture high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. We prioritize material integrity and precision production, offering custom fabrication from prototypes to high-volume orders to meet your exact specifications.
Ensure your equipment's integrity with components built for extreme conditions.
Contact our experts today to discuss your project requirements and how our PTFE solutions can benefit your application.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
People Also Ask
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability



















