In short, PTFE labware is exceptionally resistant to nearly all chemicals, including concentrated acids, bases, solvents, and aggressive cleaning agents. Its inertness makes it a default choice for chemically demanding environments. The only common exceptions are molten alkali metals and fluorine gas at high temperatures.
PTFE's remarkable chemical resistance is not a feature, but a fundamental property of its molecular structure. Understanding this allows you to trust it for virtually any application, while also knowing the specific, high-energy conditions where it will fail.

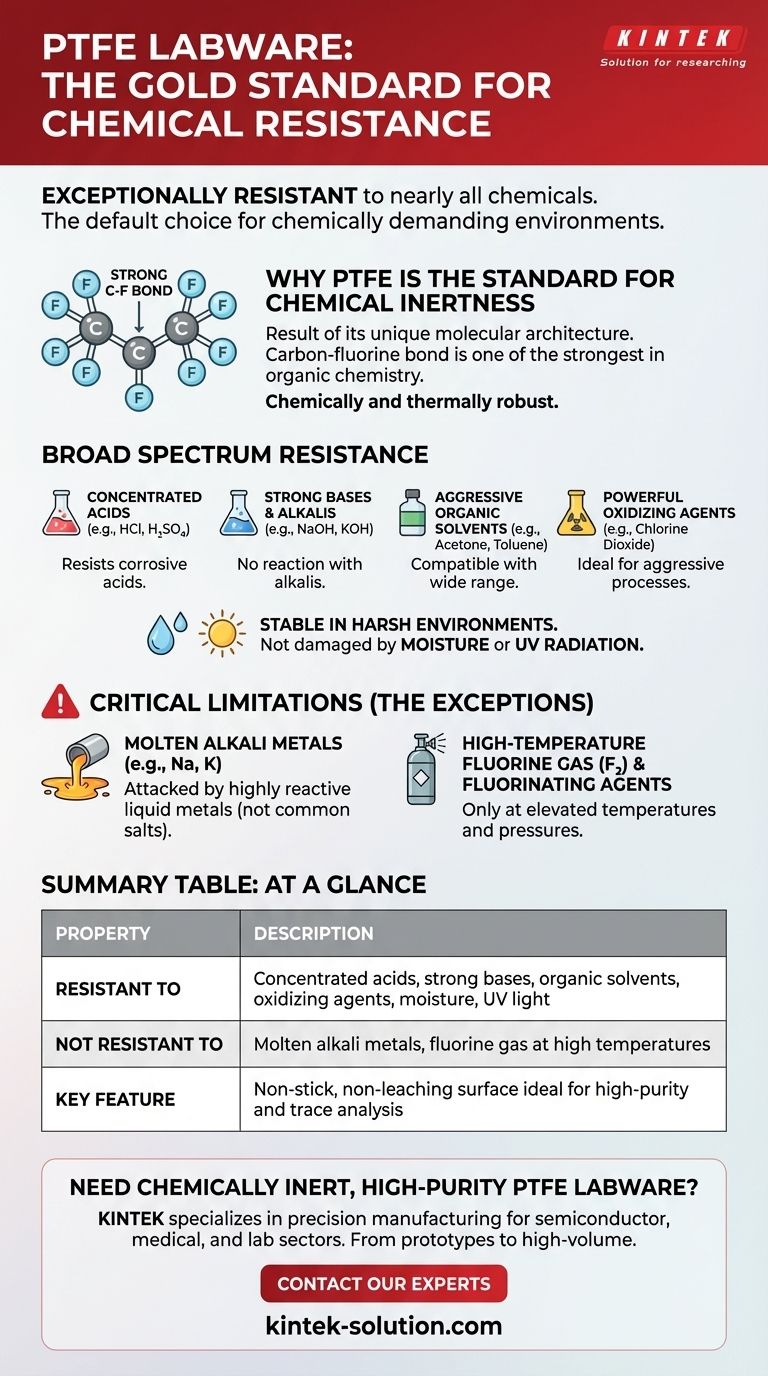
Why PTFE Is the Standard for Chemical Inertness
Polytetrafluoroethylene (PTFE) is renowned for its non-reactive nature. This inertness is not accidental; it is a direct result of its unique molecular architecture, which provides an almost impenetrable defense against chemical attack.
The Power of the Carbon-Fluorine Bond
At its core, PTFE consists of a long chain of carbon atoms completely shielded by a sheath of fluorine atoms. The carbon-fluorine bond is one of the strongest single bonds in organic chemistry.
This stable structure creates a molecule that is chemically and thermally robust. It presents no easy points of attack for other chemicals to initiate a reaction.
Broad Spectrum Resistance
Because of its stable structure, PTFE is compatible with an extremely wide range of substances. It will not react with or be degraded by:
- Concentrated and corrosive acids
- Strong bases and alkalis
- Aggressive organic solvents
- Powerful oxidizing agents like chlorine dioxide
This makes it an ideal material for beakers, containers, and tubing used in highly aggressive chemical processes.
Stability in Harsh Environments
Beyond its chemical inertness, PTFE is also highly resistant to environmental factors. It is not damaged by moisture or UV radiation, making it suitable for long-term use and storage in demanding industrial or outdoor settings.
Understanding the Critical Limitations
While its resistance is vast, PTFE is not invincible. The conditions that can compromise it are specific, rare, and typically involve very high energy states. It's crucial to know these hard boundaries to prevent catastrophic failure.
The Exception: Molten Alkali Metals
PTFE can be attacked by molten or dissolved alkali metals. This includes sodium (Na), potassium (K), and others in this group on the periodic table.
This is not a common scenario in most labs. It refers to the pure metals in their highly reactive liquid state, not their common salt forms (like sodium chloride).
The Exception: High-Temperature Fluorine
Pure fluorine gas (F₂) and certain highly reactive fluorinating agents can attack PTFE, but generally only at elevated temperatures and pressures.
This extreme reactivity allows these agents to break the powerful carbon-fluorine bonds that give PTFE its stability.
The Practical Reality
For over 99% of laboratory applications, these limitations are not a concern. The substances that attack PTFE are highly specialized and are not used in routine bench chemistry. Its non-stick, untarnishing surface remains reliable under almost all typical conditions.
Making the Right Choice for Your Application
Choosing the right labware is critical for safety and experimental integrity. PTFE's properties make it a premier choice for handling difficult substances.
- If your primary focus is general chemistry with aggressive reagents: PTFE is one of the safest and most reliable material choices available for your labware.
- If your primary focus is high-purity or trace analysis: PTFE's non-stick and non-leaching properties ensure your sample remains uncontaminated.
- If your primary focus is specialized inorganic chemistry: You must verify that your process does not involve molten alkali metals or high-temperature fluorinating agents before selecting PTFE.
Ultimately, you can confidently specify PTFE labware for nearly any chemical challenge you encounter.
Summary Table:
| Property | Description |
|---|---|
| Resistant To | Concentrated acids, strong bases, organic solvents, oxidizing agents, moisture, UV light |
| Not Resistant To | Molten alkali metals (e.g., sodium, potassium), fluorine gas at high temperatures |
| Key Feature | Non-stick, non-leaching surface ideal for high-purity and trace analysis |
Need chemically inert, high-purity PTFE labware you can trust?
KINTEK specializes in the precision manufacturing of PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Whether you need a custom prototype or a high-volume order, our expertise ensures your materials will handle even the most aggressive reagents safely.
Contact our experts today to discuss your specific application requirements.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications



















