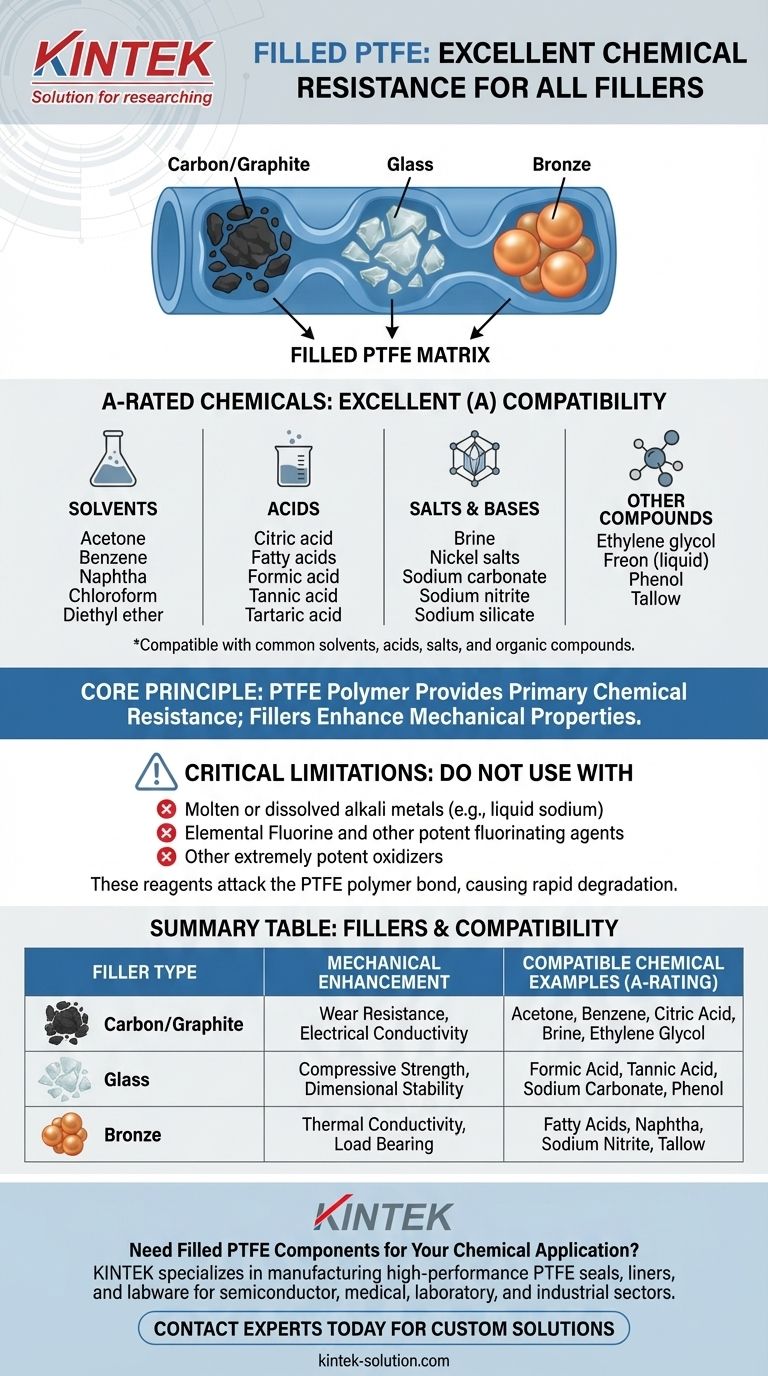
For Filled PTFE, a wide range of common industrial chemicals exhibit Excellent (A) chemical resistance across all standard filler types, including carbon/graphite, glass, and bronze. These compatible chemicals include common solvents like Acetone and Benzene, acids such as Citric and Formic acid, and various salts and organic compounds like Brine, Ethylene glycol, and Tallow.
The core principle is that Filled PTFE retains the exceptional chemical inertness of the virgin PTFE matrix. The choice of filler is typically driven by mechanical requirements, as the base polymer itself provides resistance against the vast majority of chemicals, with a few critical and well-defined exceptions.

Understanding PTFE's Chemical Compatibility
The Foundation: Inert PTFE Polymer
The remarkable chemical resistance of Filled PTFE comes from its base polymer, Polytetrafluoroethylene. This material is one of the most chemically stable plastics available.
Its molecular structure, a strong carbon-fluorine bond, makes it non-reactive to most aggressive and corrosive media, including a wide array of acids, solvents, and bases.
The Role of Fillers
Fillers like glass, carbon, and bronze are not primarily added for chemical resistance. Their purpose is to enhance mechanical properties.
For example, fillers improve wear resistance, reduce creep (cold flow), and increase compressive strength and thermal conductivity. In most service environments, these fillers are sufficiently encapsulated by the PTFE matrix to be protected.
A-Rated Chemicals: A Broad Spectrum
The chemicals rated "Excellent" for all common PTFE filler types cover a wide range of industrial applications. They can be broadly categorized:
- Solvents: Acetone, Benzene, Naphtha, Chloroform, Diethyl ether
- Acids: Citric acid, Fatty acids, Formic acid, Tannic acid, Tartaric acid
- Salts & Bases: Brine, Nickel salts, Sodium carbonate, Sodium nitrite, Sodium silicate
- Other Compounds: Ethylene glycol, Freon (liquid), Phenol, Tallow
This broad compatibility makes Filled PTFE a default choice for seals, gaskets, and bearings in many chemical processing environments.
Critical Limitations and Exceptions
Understanding where Filled PTFE fails is as important as knowing where it succeeds. The material is not universally immune, and failures in the wrong application can be catastrophic.
The Primary Weakness: Extreme Reagents
PTFE's primary vulnerability is to a very specific class of chemicals. It is not resistant and should never be used with:
- Molten or dissolved alkali metals (e.g., liquid sodium)
- Elemental Fluorine and other potent fluorinating agents
- Other extremely potent oxidizers
These substances are aggressive enough to attack the fundamental carbon-fluorine bond of the PTFE polymer itself, causing rapid degradation.
Understanding Resistance Ratings
While your focus is on "Excellent" (A) resistance, it is crucial to understand the other ratings.
A rating of "Fair" (B) suggests limited service life or that the material may swell or lose some of its properties. A rating of "Unsatisfactory" (C) means the material will degrade quickly and should not be used.
Making the Right Choice for Your Application
Your final material selection must align with both the chemical environment and the mechanical demands of the system.
- If your primary focus is compatibility with common acids, solvents, and salts: Any standard filler (carbon, glass, or bronze) is an excellent choice, so you should base your decision on mechanical needs like wear or load.
- If your environment involves any alkali metals or fluorine gas: You must avoid PTFE and all its variants entirely and seek an alternative material.
- If you are working with a chemical not on this list: Always consult a detailed chemical compatibility chart specific to Filled PTFE before proceeding, as filler material can occasionally be a factor with less common reagents.
Ultimately, confident material selection comes from understanding both the strengths and the precise, well-defined limitations of the material.
Summary Table:
| Filler Type | Key Mechanical Enhancement | Compatible Chemical Examples (Excellent 'A' Rating) |
|---|---|---|
| Carbon/Graphite | Wear Resistance, Electrical Conductivity | Acetone, Benzene, Citric Acid, Brine, Ethylene Glycol |
| Glass | Compressive Strength, Dimensional Stability | Formic Acid, Tannic Acid, Sodium Carbonate, Phenol |
| Bronze | Thermal Conductivity, Load Bearing | Fatty Acids, Naphtha, Sodium Nitrite, Tallow |
Note: The base PTFE polymer provides the primary chemical resistance. Fillers are selected for mechanical performance.
Need Filled PTFE Components for Your Chemical Application?
KINTEK specializes in manufacturing high-performance PTFE components—including seals, liners, and labware—with the precise filler (carbon, glass, or bronze) to meet your mechanical demands while ensuring excellent chemical resistance.
We serve the semiconductor, medical, laboratory, and industrial sectors with custom fabrication from prototypes to high-volume orders.
Ensure your system's reliability. Contact our experts today for a consultation on your specific chemical and mechanical requirements!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- What are the base characteristics of PTFE? Unlocking Extreme Performance in Friction, Temperature, and Chemical Resistance
- What are the primary applications of PTFE fasteners and custom parts? Critical Solutions for Extreme Environments
- What are the key benefits of PTFE in custom fabrication? Unlock Performance in Extreme Conditions
- What is PTFE commonly known as and what are its unique properties? Unlock Unmatched Chemical & Thermal Resistance
- What are the material advantages of machining Teflon? Unlock Unmatched Chemical & Thermal Resistance



















