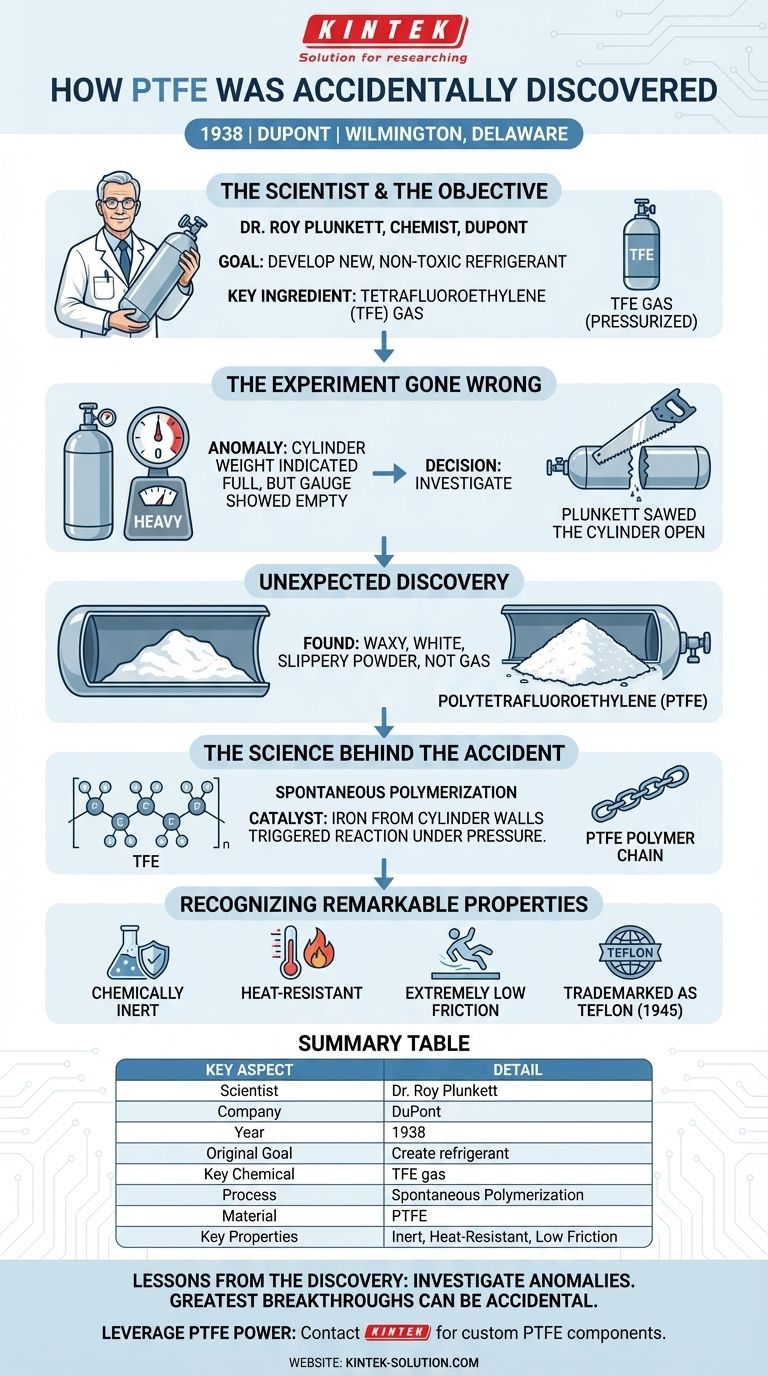
The accidental discovery of PTFE, now famously known as Teflon, occurred in 1938. Dr. Roy Plunkett, a chemist at DuPont, was attempting to create a new, non-toxic refrigerant using tetrafluoroethylene (TFE) gas. When a pressurized cylinder of the gas that should have been full stopped flowing, he investigated and found the gas had unexpectedly transformed into a mysterious white, waxy, and incredibly slippery solid.
The discovery of PTFE is a classic story of scientific serendipity. It wasn't the result of a planned experiment, but of a researcher's curiosity to understand why an experiment failed, ultimately revealing a material with world-changing properties.

The Scientist and the Objective
Dr. Roy Plunkett's Mission
In 1938, Dr. Roy Plunkett was a research chemist working for E.I. du Pont de Nemours and Company (DuPont).
His team was tasked with a clear goal: developing a new, non-toxic, and non-flammable refrigerant to replace existing compounds like ammonia and sulfur dioxide.
The Key Ingredient: TFE Gas
The specific chemical Plunkett was working with was tetrafluoroethylene (TFE), a colorless gas.
He and his assistant had produced a significant amount of TFE, which they stored in small, pressurized metal cylinders for their experiments.
The Experiment Gone Wrong
A Seemingly Empty Cylinder
The moment of discovery began with a simple anomaly. Plunkett prepared to use one of the TFE cylinders, but no gas came out when he opened the valve.
By its weight, the cylinder should have been nearly full. The pressure gauge read zero, yet the canister was far too heavy to be empty.
The Critical Decision to Investigate
Instead of discarding the apparently faulty cylinder, Plunkett’s curiosity took over. This decision was the turning point.
He and his assistant decided to saw the metal cylinder open to solve the mystery of the "disappeared" gas.
An Unexpected Discovery
Inside, they did not find gas. They found a waxy, white powder that was strangely slippery to the touch. The TFE gas had transformed into a solid.
The Science Behind the Accident
Unintended Polymerization
What had happened inside the cylinder was spontaneous polymerization. The individual TFE gas molecules (monomers) had linked together into long chains (polymers).
This process converted the gas into the solid substance now known as polytetrafluoroethylene (PTFE).
The Accidental Catalyst
The polymerization was triggered by an unexpected catalyst. The iron from the internal wall of the metal cylinder acted as the agent that kicked off the reaction, especially under the high pressure of the container.
Recognizing Remarkable Properties
Plunkett and his team quickly realized this new material wasn't a failure but a discovery. The powder was remarkably stable and inert.
It was resistant to nearly every chemical, couldn't be dissolved by any known solvent, and was extremely stable at high temperatures. Most notably, it had an incredibly low coefficient of friction, making it one of the most slippery substances known. DuPont patented the material in 1941 and registered the Teflon trademark in 1945.
Lessons from the Discovery
The story of PTFE's origin is a powerful reminder about the nature of innovation. It underscores the value of observing and investigating unexpected outcomes.
- If your primary focus is on innovation: The greatest breakthroughs can come from accidents, but only if you are prepared to recognize their potential.
- If your primary focus is on scientific process: Never dismiss an anomaly. Rigorously investigating why something went wrong is just as important as analyzing why it went right.
Ultimately, the discovery of Teflon serves as definitive proof that sometimes the most valuable results are the ones you weren't looking for.
Summary Table:
| Key Aspect | Detail |
|---|---|
| Scientist | Dr. Roy Plunkett |
| Company | DuPont |
| Year | 1938 |
| Original Goal | Create a new refrigerant |
| Key Chemical | Tetrafluoroethylene (TFE) gas |
| Accidental Process | Spontaneous polymerization inside a pressurized cylinder |
| Resulting Material | Polytetrafluoroethylene (PTFE) |
| Key Properties Found | Chemically inert, heat-resistant, extremely low friction |
Leverage the Power of PTFE in Your Projects
The accidental discovery of PTFE unlocked a material with unparalleled properties. At KINTEK, we harness these properties to manufacture high-quality, precision PTFE components—including seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors. Whether you need custom prototypes or high-volume production, our expertise ensures superior performance and reliability.
Ready to innovate with PTFE? Contact KINTEK today to discuss your specific needs and discover how our custom fabrication can benefit your application.
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- How was PTFE discovered and developed? From Lab Accident to Essential High-Performance Polymer
- What are the similarities between PTFE and RPTFE? Unlocking the Core Fluoropolymer Identity
- What is Teflon and what are its alternative names? Understanding PTFE, the Material Behind the Brand
- What are the additional properties of PTFE? Beyond Non-Stick: Extreme Chemical, Thermal & Electrical Performance
- What is PTFE commonly known as and what type of material is it? A Guide to High-Performance PTFE Properties



















