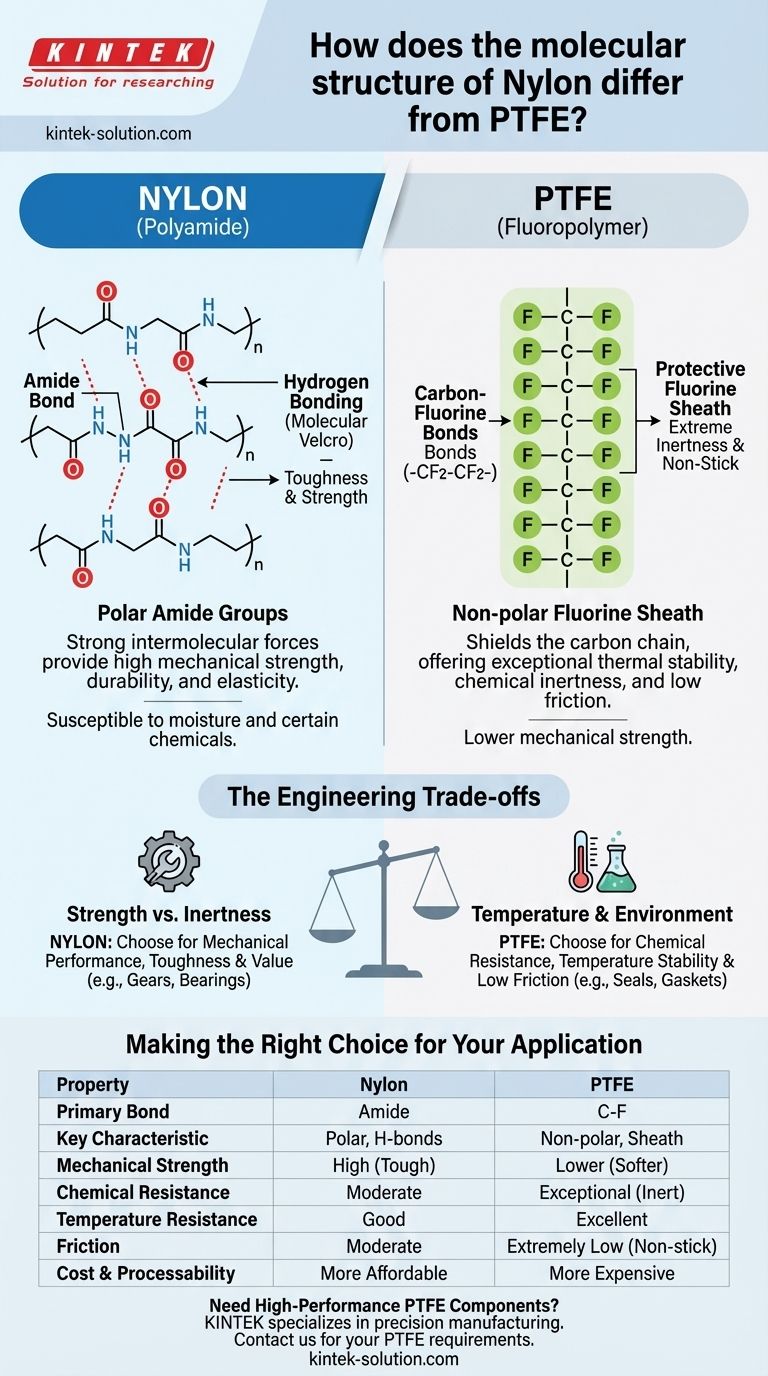
At a fundamental level, the molecular difference between Nylon and PTFE lies in their repeating chemical units. Nylon is a polyamide, characterized by strong but reactive amide bonds (-CO-NH-) that link its monomers, while PTFE (Polytetrafluoroethylene) is a fluoropolymer, defined by an incredibly stable and non-reactive backbone of carbon-fluorine bonds (-CF2-CF2-). This single distinction in bonding dictates their vastly different real-world properties.
The core difference is not just the atoms involved, but how they are arranged. Nylon's structure allows for strong intermolecular attractions (hydrogen bonds) that give it mechanical strength, whereas PTFE's structure creates a protective atomic "sheath" that gives it extreme chemical inertness and a non-stick surface.

The Core of Nylon: The Amide Bond
Nylon’s properties are a direct result of the repeating amide groups in its polymer chain. This structure is what makes it a premier material for mechanical components.
The Polyamide Chain Structure
Nylon is built from chains containing repeating amide groups (-CO-NH-). These groups are polar, meaning they have a slight electrical charge separation.
This inherent polarity is the key to understanding all of Nylon's mechanical characteristics.
The Role of Hydrogen Bonding
The polarity of the amide groups allows adjacent polymer chains to attract each other strongly through hydrogen bonds. The hydrogen atom on one amide group is attracted to the oxygen atom on a neighboring chain.
These numerous, chain-to-chain hydrogen bonds act like molecular-level velcro, locking the chains together and giving the bulk material its signature toughness and strength.
Implications for Material Properties
This interconnected molecular structure gives Nylon high tensile strength, durability, and elasticity. The chains can stretch and move but are constantly pulled back by the powerful hydrogen bonds.
However, this same structure is susceptible to moisture absorption and attack from certain chemicals, which can disrupt the hydrogen bonding network.
The Fortress of PTFE: The Carbon-Fluorine Bond
PTFE’s structure is simpler than Nylon’s, but its properties are more extreme. Its identity is defined by the unique relationship between carbon and fluorine.
The Fluoropolymer Structure
PTFE, known commercially as Teflon®, has a very simple, linear backbone of carbon atoms. The defining feature is that each carbon atom is bonded to two fluorine atoms.
The carbon-fluorine (C-F) bond is one of the strongest single bonds known in organic chemistry.
The Protective Fluorine Sheath
Because fluorine atoms are relatively large and highly electronegative, they form a tight, continuous, and non-polar sheath around the carbon backbone.
This fluorine sheath effectively shields the more vulnerable carbon chain from any chemical attack. It leaves no "handholds" for other molecules to latch onto, which is the source of PTFE's famous non-stick property and low friction.
Implications for Material Properties
The immense strength of the C-F bonds and the protective sheath give PTFE exceptional thermal stability and near-universal chemical inertness.
Its electrically stable and symmetrical fluorine sheath also makes it an excellent electrical insulator. The material is dense and has high crystallinity.
Understanding the Engineering Trade-offs
Choosing between Nylon and PTFE is a classic engineering decision that balances mechanical performance against environmental resistance.
Strength vs. Inertness
Nylon's primary advantage is its mechanical strength and wear resistance due to hydrogen bonding. PTFE’s primary advantage is its chemical inertness and low friction due to the fluorine sheath.
You choose Nylon when you need a part to be tough and durable. You choose PTFE when a part must survive harsh chemicals or have a slippery surface.
Temperature and Environment
Nylon performs well in moderate temperature environments but can be degraded by strong acids and moisture.
PTFE excels in extreme temperature applications, both high and low, and is virtually unaffected by any common solvent or corrosive agent.
Cost and Manufacturing
Nylon is significantly more affordable and easier to process than PTFE, making it a go-to choice for a vast range of high-volume parts, from gears to fabric fibers.
PTFE is a more expensive specialty polymer that requires specific manufacturing processes, reserving it for applications where its unique properties are indispensable.
Making the Right Choice for Your Application
Your application's environment and performance requirements will dictate which material is the correct choice.
- If your primary focus is mechanical performance and cost-effectiveness: Choose Nylon for its excellent strength, toughness, and value in applications like gears, bearings, or structural components.
- If your primary focus is chemical resistance, temperature stability, or low friction: Choose PTFE for its unrivaled inertness and non-stick properties in applications like seals, gaskets, non-stick coatings, or chemical linings.
Ultimately, understanding the molecular structure of these polymers removes the guesswork and empowers you to select the material that is perfectly suited to its function.
Summary Table:
| Property | Nylon (Polyamide) | PTFE (Fluoropolymer) |
|---|---|---|
| Primary Bond | Amide Bond (-CO-NH-) | Carbon-Fluorine Bond (-CF2-) |
| Key Characteristic | Polar, forms hydrogen bonds | Non-polar, forms a protective sheath |
| Mechanical Strength | High (Tough, Durable) | Lower (Softer) |
| Chemical Resistance | Moderate (Susceptible to moisture/chemicals) | Exceptional (Inert to most chemicals) |
| Temperature Resistance | Good (Moderate temperatures) | Excellent (Extreme high/low temperatures) |
| Friction | Moderate | Extremely Low (Non-stick) |
| Cost & Processability | More affordable, easier to process | More expensive, specialized processing |
Need High-Performance PTFE Components?
Understanding the molecular science is the first step. Applying it is the next. KINTEK specializes in the precision manufacturing of PTFE components—including seals, liners, and custom labware—for industries where chemical inertness, purity, and thermal stability are non-negotiable.
We serve the semiconductor, medical, laboratory, and industrial sectors with custom fabrication from prototypes to high-volume orders. Let us provide the material solution that is perfectly suited to your function.
Contact KINTEK today to discuss your PTFE requirements.
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- How does PTFE react to common solvents? Discover Its Near-Total Chemical Immunity
- What is Teflon and what are its alternative names? Understanding PTFE, the Material Behind the Brand
- What is PTFE and what class of plastics does it belong to? A Guide to High-Performance Fluoropolymers
- What is PTFE commonly known as and what type of material is it? A Guide to High-Performance PTFE Properties
- What is Teflon and what is its chemical name? Unpacking the Science of PTFE



















