At its core, PTFE's non-stick quality is due to its unique molecular architecture: a carbon backbone completely encased in a tight, uniform sheath of fluorine atoms. This fluorine "armor" creates an exceptionally stable and low-energy surface that is chemically non-reactive. Because other substances have nothing to chemically "grab onto," they simply slide off.
The non-stick property of Polytetrafluoroethylene (PTFE) is not an applied coating, but an inherent result of its molecular structure. The strong, stable fluorine-carbon bonds create a chemically inert and low-energy surface that repels nearly all other substances on a molecular level.

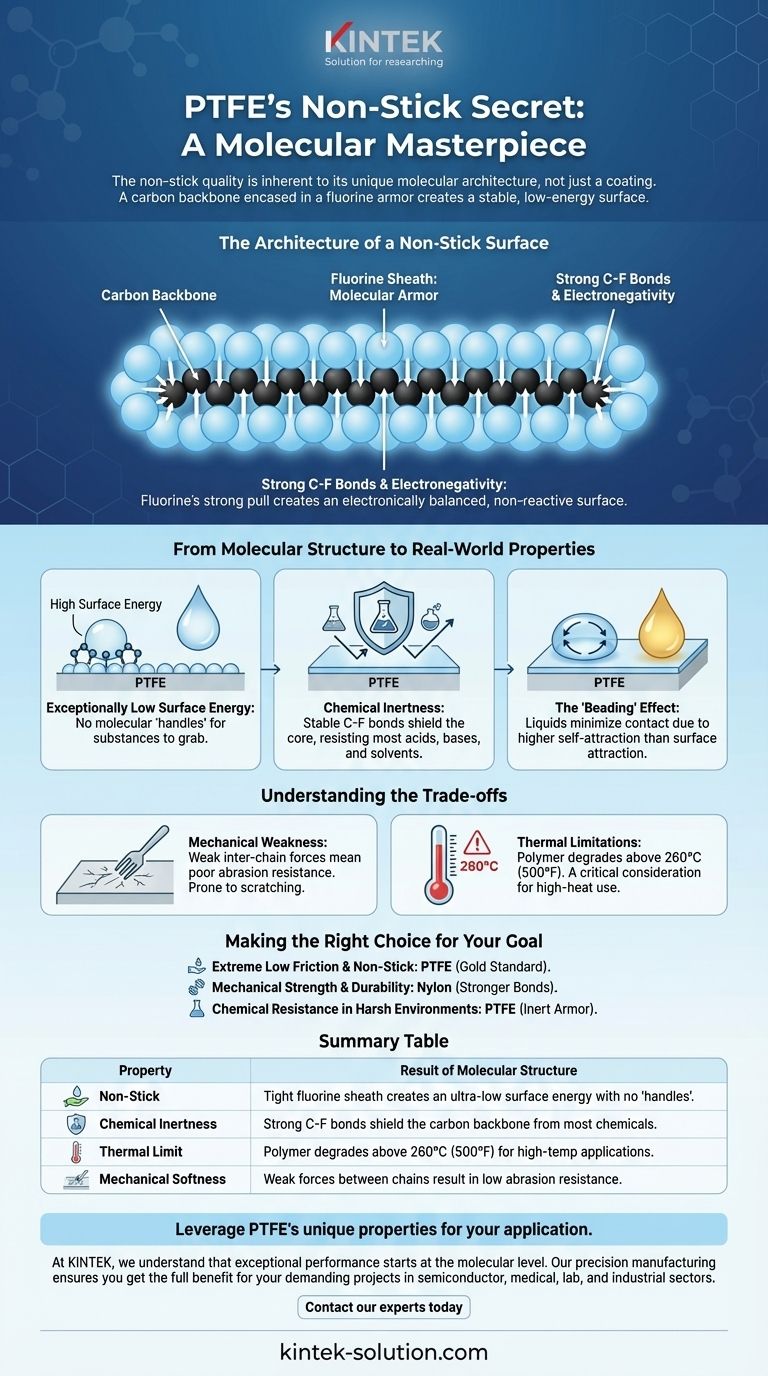
The Architecture of a Non-Stick Surface
To understand PTFE's behavior, we must first examine its construction at the atomic scale. The properties emerge directly from this fundamental design.
The Carbon Backbone
Like many polymers, PTFE starts with a long chain of carbon atoms bonded together. This chain provides the structural spine for the entire molecule.
The Fluorine Sheath: Molecular Armor
The key to PTFE is what's attached to that carbon backbone. Every carbon atom is bonded to two fluorine atoms, which are relatively large. These fluorine atoms pack so tightly together they form a seamless, protective sheath around the entire carbon chain.
This protective layer is held together by some of the strongest single bonds in organic chemistry: the carbon-fluorine (C-F) bond. This makes the sheath incredibly stable and difficult to break apart.
Electronegativity and Repulsion
Fluorine is the most electronegative element, meaning it has an extremely strong pull on its electrons. This creates a very stable, electronically balanced surface with no weak spots for other molecules to interact with. The result is a surface that is profoundly non-reactive.
From Molecular Structure to Real-World Properties
This unique atomic arrangement directly translates into the macroscopic properties that make PTFE so useful.
Exceptionally Low Surface Energy
The fluorine sheath gives PTFE one of the lowest surface energies of any known solid. Think of surface energy as a measure of "molecular stickiness." Surfaces with high energy have molecular "handles" that other substances can grab.
PTFE has virtually no handles. This lack of attraction is the direct cause of its non-stick nature.
Chemical Inertness
Because the carbon backbone is completely shielded by the stable fluorine atoms and strong C-F bonds, it is nearly impossible for other chemicals to attack it. This makes PTFE resistant to almost all acids, bases, and solvents.
The "Beading" Effect
When a liquid like water or oil is placed on PTFE, its own molecules are more attracted to each other than they are to the PTFE surface. This forces the liquid to minimize its contact with the surface, resulting in the characteristic "beading" and rolling effect.
Understanding the Trade-offs
No material is perfect. The same molecular structure that provides incredible non-stick properties also introduces limitations.
Mechanical Weakness
The forces holding individual PTFE polymer chains together are relatively weak. This makes the material soft and gives it poor abrasion resistance. It's why non-stick coatings can be easily scratched by metal utensils.
Thermal Limitations
While the C-F bonds are very strong, the overall polymer can begin to degrade at temperatures above 260°C (500°F). This is a critical consideration in applications like cookware, as overheating can compromise the material's integrity.
Making the Right Choice for Your Goal
Understanding PTFE's molecular foundation allows you to select materials based on your primary objective.
- If your primary focus is extreme low friction and non-stick performance: PTFE is the gold standard precisely because its fluorine sheath creates a uniquely low-energy, non-reactive surface.
- If your primary focus is mechanical strength and durability: A material like Nylon, with its strong inter-chain amide bonds, would be a far superior choice over the softer PTFE.
- If your primary focus is chemical resistance in a harsh environment: PTFE's inert molecular armor makes it one of the best possible choices for handling corrosive substances.
Ultimately, understanding how a material's atomic structure dictates its function is the key to true engineering insight.
Summary Table:
| Property | Result of Molecular Structure |
|---|---|
| Non-Stick | Tight fluorine sheath creates an ultra-low surface energy with no 'handles' for other molecules to grab. |
| Chemical Inertness | Strong carbon-fluorine bonds shield the carbon backbone from nearly all acids, bases, and solvents. |
| Thermal Limit | Polymer begins to degrade above 260°C (500°F), a key consideration for high-temperature applications. |
| Mechanical Softness | Weak forces between polymer chains result in low abrasion resistance, making it prone to scratching. |
Leverage PTFE's unique properties for your application.
At KINTEK, we understand that the exceptional performance of PTFE components—from seals and liners to custom labware—starts at the molecular level. Our precision manufacturing ensures you get the full benefit of PTFE's non-stick and chemically inert nature for your most demanding projects in the semiconductor, medical, laboratory, and industrial sectors.
Whether you need prototypes or high-volume orders, our expertise in custom fabrication means we can deliver the exact PTFE solution your design requires.
Contact our experts today to discuss how our PTFE components can solve your specific challenges.
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- What are the advantages of using PTFE Lip Seals in high-speed rotary applications? Achieve Superior Speed and Reliability
- What are the key advantages of PTFE rotary seals over traditional rubber seals? Superior Performance in Extreme Conditions
- What are the limitations of PTFE bellows? Understanding Its Mechanical and Chemical Boundaries
- What control options are available for PTFE/PFA lined ball valves? Choose the Right Actuation Method
- How do PTFE and NBR oil seals compare in terms of temperature resistance? Choose the Right Seal for Extreme Heat
- How does FR4 PCB material compare to PTFE in terms of electrical properties? Choose the Right Material for Your Application.
- In which industries is porous PTFE applied? Solve Harsh Environment Challenges with KINTEK
- What makes PTFE V-rings suitable for sealing applications? Superior Performance in Extreme Conditions



















