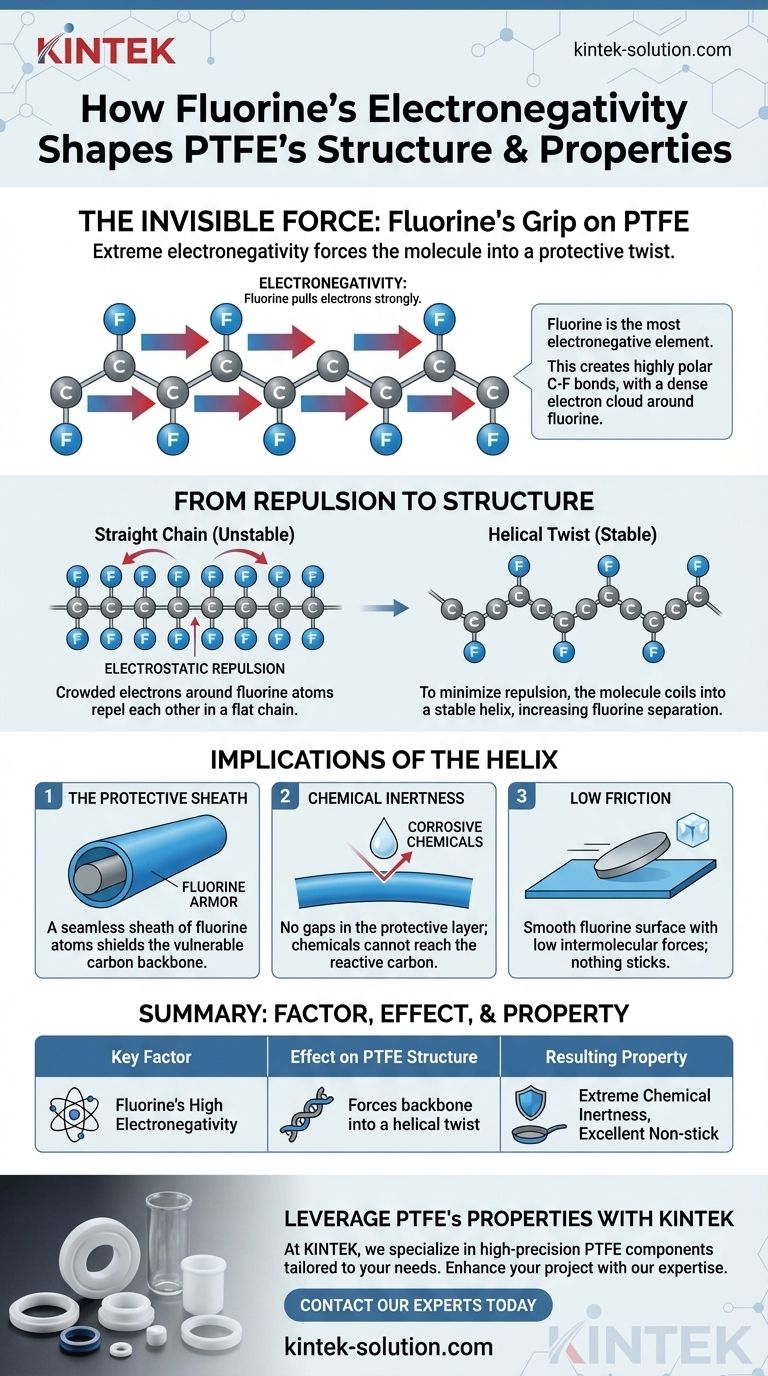
In short, fluorine's extreme electronegativity forces the PTFE molecule into a protective twist. It pulls electrons so strongly from the central carbon chain that the resulting fluorine atoms repel each other, forcing the entire polymer backbone to coil into a tight, stable helix.
The core principle to understand is that an electrical property—electronegativity—directly dictates a physical outcome. The powerful electrostatic repulsion between fluorine atoms is the architect of PTFE's unique helical structure, which in turn is the source of its famous properties.

The Foundation: Electronegativity and the C-F Bond
To grasp PTFE's structure, we must first start with the powerful and unique nature of the carbon-fluorine (C-F) bond.
Fluorine's Unmatched Electron Affinity
Fluorine is the most electronegative element on the periodic table. This means it has an unparalleled ability to attract bonding electrons to itself.
Creating a Highly Polar Bond
When bonded to carbon, fluorine's immense electronegativity pulls the shared electrons so strongly that it creates a significant partial negative charge on the fluorine atom and a partial positive charge on the carbon atom. This results in a very strong and highly polar C-F bond.
From Electrical Repulsion to Physical Structure
This intense bond polarity has profound consequences for the overall shape of the long polymer chain.
The Crowded Electron Shield
Each carbon atom in the PTFE backbone is bonded to two fluorine atoms. Because of the C-F bond's polarity, this creates a dense, negatively charged shield of electrons completely surrounding the carbon skeleton.
Why a Straight Chain is Unstable
If the PTFE molecule tried to form a simple, flat "zigzag" chain (similar to polyethylene), these large, electron-rich fluorine atoms would be forced too close together. The resulting electrostatic repulsion would make this planar structure highly unstable.
The Helical Solution
To relieve this strain, the molecule twists. The C-C backbone rotates slightly at each bond, forcing the fluorine atoms into a helical, or spiral, configuration. This twist elegantly increases the distance between the fluorine atoms, minimizing repulsion and settling the molecule into a low-energy, highly stable state.
The Implications of a Helical Structure
This unique shape is not just a chemical curiosity; it is the direct cause of PTFE's most valued characteristics.
The Protective Fluorine Sheath
The helical structure creates a seamless, tightly packed sheath of fluorine atoms around the vulnerable carbon backbone. There are no gaps or weaknesses in this protective layer.
The Root of Chemical Inertness
Because the chemically reactive carbon chain is perfectly shielded, other chemicals simply cannot reach it to react. This fluorine "armor" is what makes PTFE one of the most chemically inert substances known.
The Origin of Low Friction
The surface of the PTFE molecule is a smooth, uniform layer of fluorine atoms with very low intermolecular forces. These fluorine atoms have their electrons held so tightly that they have little incentive to interact with other molecules, which is why almost nothing sticks to PTFE.
How to Apply This Knowledge
Understanding this fundamental link between electronegativity and structure allows you to predict and explain PTFE's behavior.
- If your primary focus is chemical resistance: Recognize that the helical fluorine sheath, a direct result of electron repulsion, physically blocks corrosive agents from reaching the carbon backbone.
- If your primary focus is non-stick performance: Know that the stable, low-energy electron shield on the molecule's surface offers no purchase for other materials to form chemical bonds.
- If your primary focus is thermal stability: Appreciate that the fundamental strength of the carbon-fluorine bond itself is what allows PTFE to withstand high temperatures without degrading.
Ultimately, PTFE's remarkable properties are a direct and elegant consequence of fluorine's fundamental atomic character.
Summary Table:
| Key Factor | Effect on PTFE Structure | Resulting Property |
|---|---|---|
| Fluorine's High Electronegativity | Creates strong, polar C-F bonds | High thermal stability |
| Electrostatic Repulsion | Forces backbone into a helical twist | Seamless fluorine sheath |
| Helical Configuration | Forms a protective atomic shield | Extreme chemical inertness |
| Smooth Fluorine Surface | Low intermolecular forces | Excellent non-stick performance |
Leverage PTFE's Superior Properties for Your Application
Understanding the fundamental science behind PTFE's structure is key to selecting the right material for demanding environments. At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—that harness these unique properties for optimal performance.
Whether you're in the semiconductor, medical, laboratory, or industrial sector, our expertise in custom fabrication ensures you get components tailored to your specific needs, from prototypes to high-volume production.
Ready to enhance your project with PTFE's exceptional chemical resistance and non-stick capabilities? Contact our experts today to discuss your requirements and discover how KINTEK's precision PTFE solutions can benefit your operation.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- How does graphite-filled PTFE perform? A Guide to Superior Self-Lubricating Components
- What are the key material properties of PTFE? Unlock Superior Performance for Demanding Applications
- What are the two common forms in which PTFE is available? Raw Resins vs. Semi-Finished Shapes
- What temperature range can PTFE withstand? Master Its -200°C to 260°C Operating Window
- How is Teflon made? The Science Behind Its Incredible Properties
- What quality control measures are used in PTFE production? Ensure Material Integrity for Your Application
- Why is PTFE known for its non-stick properties? The Science Behind Its Slippery Surface
- What are the properties and applications of carbon-filled PTFE? Enhance Performance in Demanding Environments



















