In short, Polytetrafluoroethylene (PTFE) exhibits extraordinary resistance to virtually all common solvents. Even at elevated temperatures and over long periods of exposure, it shows minimal absorption or degradation. This near-total chemical inertness is a defining characteristic of the material, making it a benchmark for performance in corrosive environments.
The core reason for PTFE's extreme solvent resistance lies in its molecular structure. The exceptionally strong carbon-fluorine bonds are highly stable and non-polar, creating a chemically inert and hydrophobic surface that repels nearly all substances.

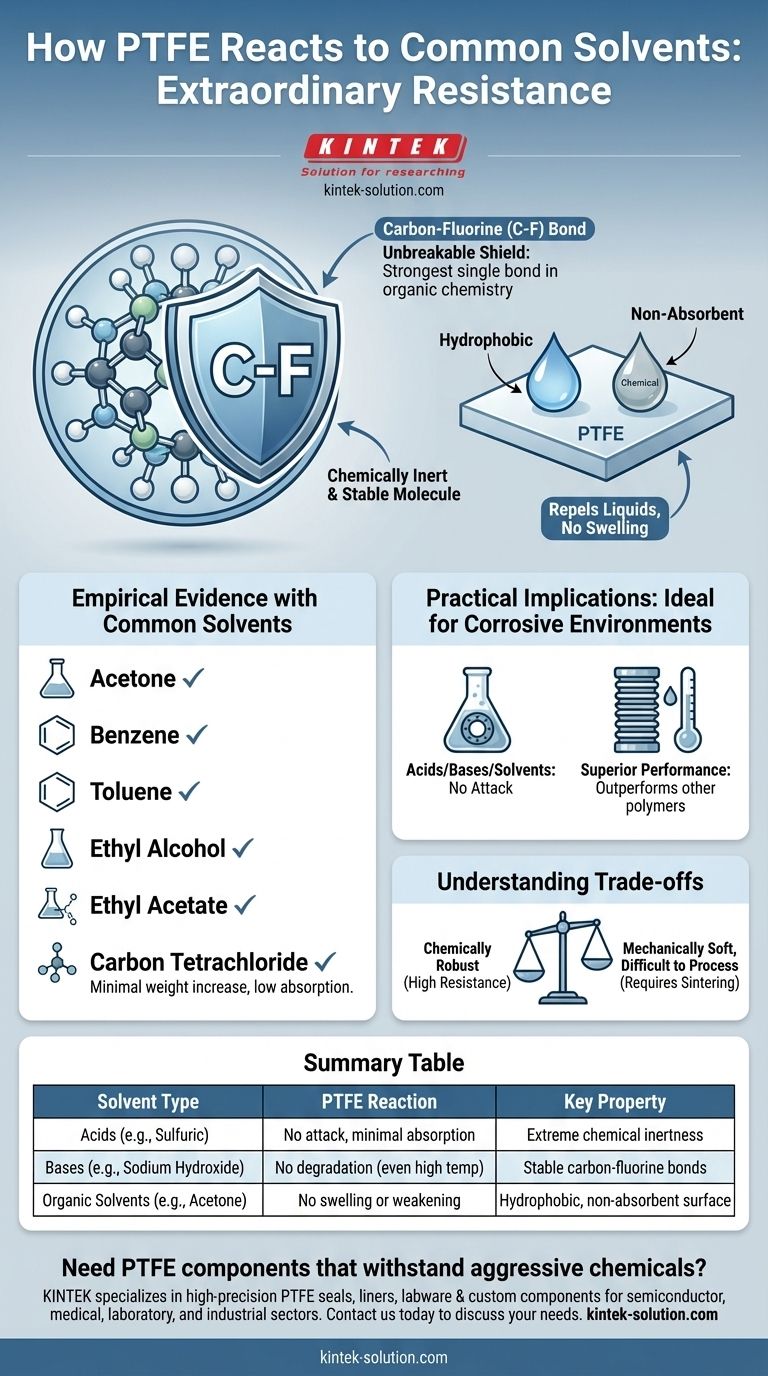
The Foundation of PTFE's Chemical Inertness
To understand why PTFE is so resilient, we must look at its fundamental chemical and physical properties. It is not merely a coating; its entire structure is built for non-reactivity.
The Carbon-Fluorine Bond: An Unbreakable Shield
The backbone of PTFE consists of a chain of carbon atoms completely sheathed by fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry.
This powerful bond creates a highly stable molecule that is extremely difficult for chemical solvents to attack or break down. This is the primary source of its virtual immunity to chemical attack.
Hydrophobic and Non-Absorbent Nature
PTFE is profoundly hydrophobic, meaning it actively repels water. This property extends to most other liquids as well.
Because liquids do not adhere to its surface and are not absorbed into its structure, solvents cannot penetrate the material to cause swelling, weakening, or degradation. This contributes to its excellent performance as a barrier material.
Empirical Evidence with Common Solvents
Testing confirms this high level of resistance across a wide range of chemicals.
When exposed to solvents such as acetone, benzene, carbon tetrachloride, ethyl alcohol, ethyl acetate, and toluene, PTFE shows only a minimal increase in weight. This demonstrates its low absorption and stability even when challenged by aggressive organic compounds.
Practical Implications of Extreme Resistance
This unique chemical resilience makes PTFE indispensable in many demanding industrial and scientific applications where other materials would quickly fail.
Ideal for Corrosive Environments
PTFE is virtually immune to attack from nearly all acids, bases, and organic solvents. This makes it the material of choice for gaskets, seals, linings, and tubing that handle highly corrosive substances.
Superior Performance in Demanding Applications
Compared to other polymers, PTFE is often in a class of its own. In applications like flexible chemical bellows, it outperforms all other materials due to its status as the most chemically inert polymer available.
Self-Cleaning and Barrier Integrity
Because liquids bead up and run off its surface, PTFE is considered self-cleaning. This ensures that residues do not build up and compromise processes, a critical feature in high-purity applications.
Understanding the Trade-offs
While its chemical resistance is nearly absolute, it is important to consider PTFE within the full context of engineering requirements. Its unique properties present certain trade-offs.
Chemical vs. Physical Properties
While chemically robust, PTFE is a relatively soft material. Design considerations often center on its mechanical properties, such as compressive strength, creep (deformation under load), and abrasion resistance, more than its chemical limits.
Processing Challenges
The same chemical inertness and high melting point that make PTFE so durable also make it difficult to process. It cannot be melt-processed like common plastics, requiring specialized techniques like sintering.
Near-Universal, Not Absolute, Immunity
The term "virtually immune" is accurate. While PTFE resists the vast majority of chemicals, a few highly reactive substances (such as molten alkali metals and certain fluorine compounds at high temperatures) can attack it. For common solvents, however, its resistance is effectively total.
Making the Right Choice for Your Application
Leveraging PTFE correctly means matching its unique strengths to your primary goal.
- If your primary focus is handling aggressive chemicals: Use PTFE for components that will be in direct contact with a wide range of corrosive acids, bases, or organic solvents.
- If your primary focus is preventing contamination: Its hydrophobic and non-absorbent surface makes it ideal for high-purity systems where leaching or material absorption is unacceptable.
- If your primary focus is performance at high temperatures: Select PTFE for applications where solvent resistance must be maintained at elevated process temperatures, as its stability is not compromised.
Ultimately, when your application demands unwavering resilience against chemical attack, PTFE remains the definitive material choice.
Summary Table:
| Solvent Type | PTFE Reaction | Key Property |
|---|---|---|
| Acids (e.g., Sulfuric, Hydrochloric) | No attack, minimal absorption | Extreme chemical inertness |
| Bases (e.g., Sodium Hydroxide) | No degradation, even at high temperatures | Stable carbon-fluorine bonds |
| Organic Solvents (e.g., Acetone, Toluene) | No swelling or weakening | Hydrophobic, non-absorbent surface |
Need PTFE components that withstand aggressive chemicals?
At KINTEK, we specialize in manufacturing high-precision PTFE seals, liners, labware, and custom components for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment maintains barrier integrity and purity in corrosive environments.
Contact us today to discuss your custom fabrication needs—from prototypes to high-volume orders.
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What is PTFE commonly known as and what type of material is it? A Guide to High-Performance PTFE Properties
- What is PTFE and what class of plastics does it belong to? A Guide to High-Performance Fluoropolymers
- What is Teflon and what is its chemical name? Unpacking the Science of PTFE
- What is Teflon and what are its alternative names? Understanding PTFE, the Material Behind the Brand
- What environmental resistances does PTFE offer? Unmatched Durability for Harsh Conditions



















