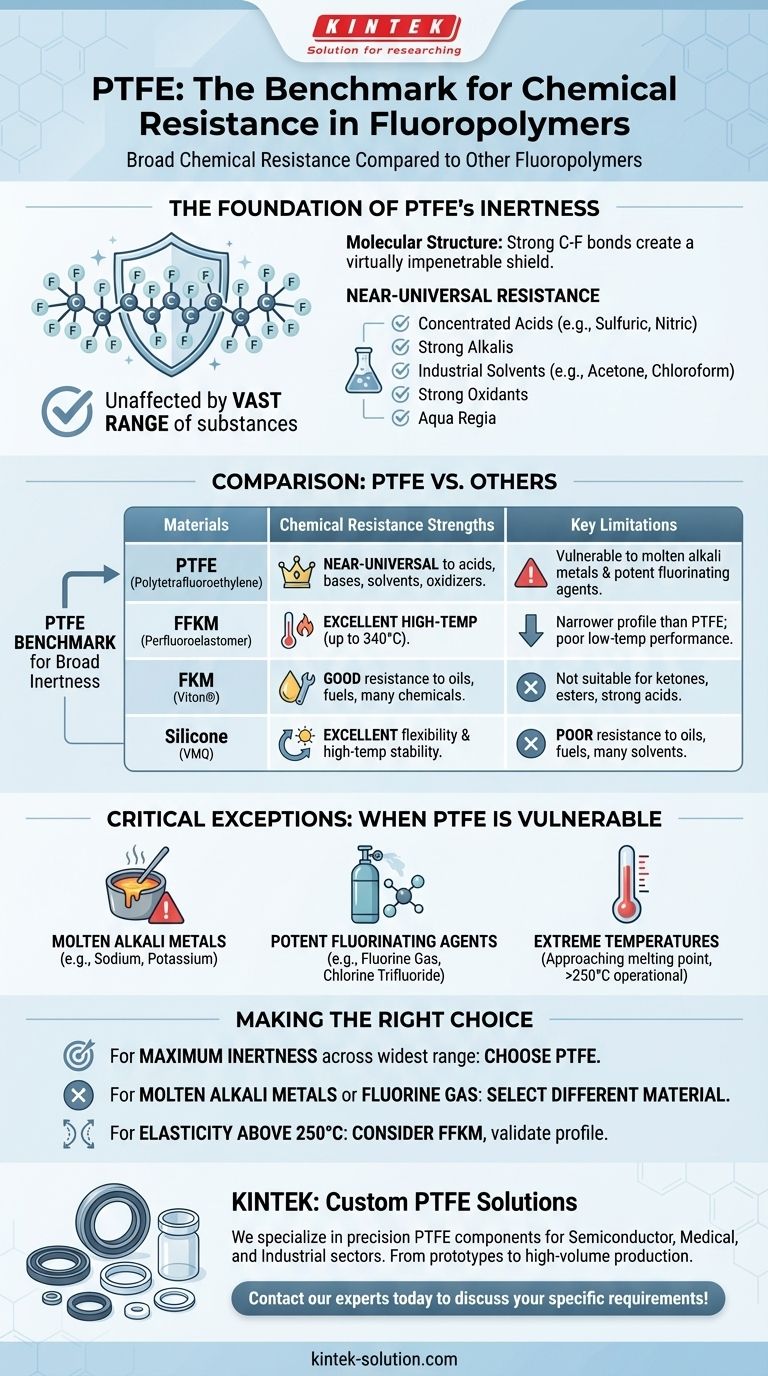
In terms of broad chemical resistance, Polytetrafluoroethylene (PTFE) is a benchmark material. It is one of the most chemically inert polymers known, outperforming nearly all other fluoropolymers and high-performance plastics when faced with aggressive acids, solvents, and corrosive agents. Its performance is so comprehensive that it is often the default choice for the most demanding chemical applications.
The core takeaway is that PTFE offers near-universal chemical resistance, making it superior to most materials. However, its effectiveness has clear and critical exceptions: it will be attacked by molten alkali metals, fluorine gas, and a few related fluorine compounds.

The Foundation of PTFE's Chemical Inertness
PTFE's exceptional performance stems directly from its molecular structure. The strong, stable bonds between carbon and fluorine atoms create a protective shield that is incredibly difficult for other chemicals to break.
Near-Universal Resistance
This molecular stability makes PTFE virtually impervious to a vast range of substances. It remains unaffected by concentrated and boiling acids like sulfuric, nitric, and hydrochloric acid.
It also resists strong alkalis, strong oxidants, and nearly all industrial organic solvents, including acetone and chloroform. Materials like aqua regia, famous for dissolving noble metals, have no effect on PTFE.
Superiority Over Other Plastics
When compared to other high-performance plastics, PTFE's chemical resistance is in a class of its own. It significantly outperforms materials like PEEK, Nylon, and Polyethylene (PE), especially in harsh chemical environments and at elevated temperatures.
How PTFE Compares to Sealing Elastomers
In applications like seals and O-rings, the comparison between PTFE and various elastomers highlights its unique advantages and specific limitations.
Against FKM, EPDM, and Silicone
While elastomers like FKM (Viton®), EPDM, and Silicone (VMQ) offer flexibility, their chemical resistance is narrower than PTFE's. Silicone, for example, may match PTFE's high-temperature performance but cannot withstand the same breadth of chemicals.
Against Perfluoroelastomers (FFKM)
FFKM is one of the few materials that can compete with or even exceed PTFE in specific areas. It can handle higher temperatures (up to 340°C) but typically has poorer performance at very low temperatures. While its chemical resistance is also exceptional, PTFE remains the benchmark for broad, near-universal inertness at its operational temperatures.
Understanding the Trade-offs: The Few Exceptions
No material is perfect, and building trust requires understanding a material's precise limitations. PTFE's near-total inertness has a few, very specific vulnerabilities that are critical to recognize.
Molten Alkali Metals
PTFE will react with and be degraded by contact with molten or dissolved alkali metals, such as sodium or potassium.
Potent Fluorinating Agents
High-pressure fluorine gas and related compounds like chlorine trifluoride are among the only chemicals that will attack the PTFE polymer chain under certain conditions.
Extreme Temperatures
While PTFE is highly insoluble, its properties can be affected at temperatures approaching its melting point (around 327°C). Its primary operational temperature range is generally cited as -250°C to +250°C.
Making the Right Choice for Your Application
Selecting the correct material requires matching its profile to the specific demands of your environment.
- If your primary focus is maximum chemical inertness across the widest range of acids, bases, and solvents: PTFE is the definitive and most reliable choice.
- If your application involves molten alkali metals, fluorine gas, or chlorine trifluoride: You must select a different material, as PTFE is unsuitable for these specific media.
- If you require elasticity or sealing performance at temperatures consistently above 250°C (482°F): Investigate specialized materials like FFKM, but validate its performance against your specific chemical and temperature profile.
Ultimately, PTFE's remarkable chemical resistance makes it the standard against which other polymers are often measured.
Summary Table:
| Material | Key Chemical Resistance Strengths | Key Limitations |
|---|---|---|
| PTFE | Near-universal resistance to acids, bases, solvents, and oxidizers. | Vulnerable to molten alkali metals and potent fluorinating agents. |
| FFKM | Excellent high-temperature resistance (up to 340°C). | Narrower chemical profile than PTFE; poor low-temperature performance. |
| FKM (Viton®) | Good resistance to oils, fuels, and many chemicals. | Not suitable for ketones, esters, or strong acids. |
| Silicone | Excellent flexibility and high-temperature stability. | Poor resistance to oils, fuels, and many solvents. |
Need a component that can withstand your most aggressive chemical processes?
At KINTEK, we specialize in manufacturing precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, and industrial sectors. Our expertise in custom fabrication ensures you get the exact chemical resistance and performance your application demands, from prototypes to high-volume production.
Contact our experts today to discuss your specific requirements and get a solution tailored for maximum durability and chemical inertness.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- In which industries is PTFE commonly used? Key Applications for Chemical & Thermal Resistance
- What industrial applications does PTFE have? Unlock Performance in Extreme Environments
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE



















