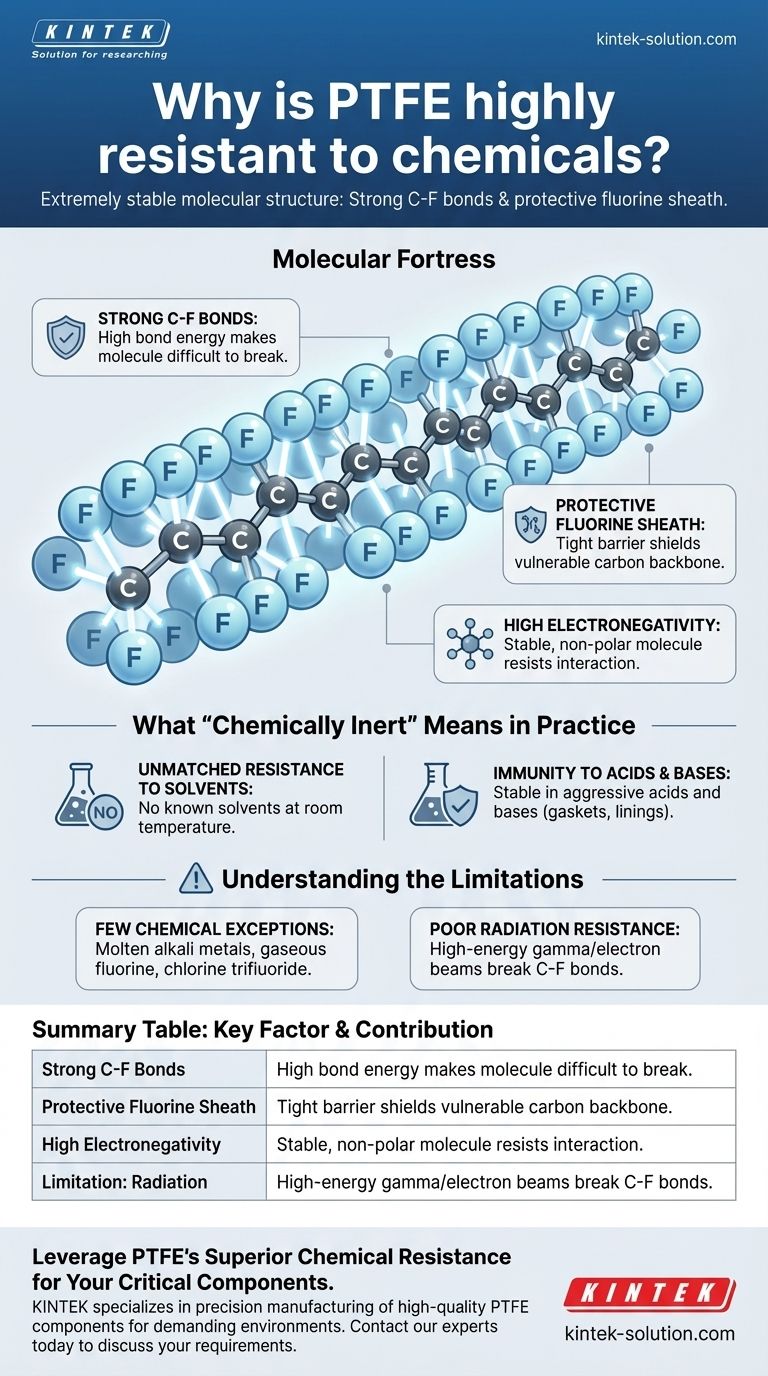
The remarkable chemical resistance of PTFE stems from its unique and exceptionally stable molecular structure. At its core are the powerful chemical bonds between carbon and fluorine atoms, which are the strongest single bonds in organic chemistry, making the molecule incredibly difficult for other chemicals to break apart.
The core reason for PTFE's inertness is twofold: extremely strong carbon-fluorine bonds that are difficult to break, and a tightly packed "sheath" of fluorine atoms that physically protects the vulnerable carbon backbone from chemical attack.

The Molecular Fortress: Deconstructing PTFE's Structure
To understand PTFE's resilience, we must look at how it is built at the atomic level. Its properties are not accidental; they are a direct result of its chemical makeup.
The Carbon-Fluorine (C-F) Bond
The C-F bond is the defining feature of PTFE. It requires an immense amount of energy to sever this connection, far more than most chemical reactions can provide. This inherent strength makes the molecule highly non-reactive.
The Protective Fluorine Sheath
The fluorine atoms are significantly larger than the carbon atoms they surround. They pack tightly around the carbon backbone, creating a uniform, helical sheath. This physical barrier effectively blocks corrosive agents from ever reaching and attacking the carbon chain.
High Electronegativity
Fluorine is the most electronegative element, meaning it holds onto its electrons very tightly. This creates a very stable, non-polar molecule that does not easily interact with other substances, contributing to its inertness.
What "Chemically Inert" Means in Practice
This molecular stability translates into tangible, real-world benefits that make PTFE a critical material in demanding industries.
Unmatched Resistance to Solvents
PTFE is famously insoluble. There are no known solvents that can dissolve it at or near room temperature. This makes it an ideal material for containers and tubing that handle a wide variety of chemical mixtures.
Immunity to Acids and Bases
The material remains completely stable when exposed to nearly all aggressive acids and bases. This is why it is used extensively for gaskets, seals, and linings in chemical processing equipment where other materials would quickly degrade.
Understanding the Limitations
While often called the most chemically resistant plastic, PTFE is not invincible. Understanding its specific vulnerabilities is crucial for proper application and safety.
The Few Chemical Exceptions
Only a handful of extremely reactive substances are known to attack PTFE. These are typically not encountered outside of highly specialized industrial or laboratory settings and include molten alkali metals (like sodium), gaseous fluorine, and potent fluorinating agents like chlorine trifluoride.
Poor Radiation Resistance
A significant trade-off for PTFE's chemical stability is its poor resistance to high-energy radiation. Gamma rays or electron beams can break the C-F bonds, causing the molecular structure to break down and the material to lose its integrity.
Making the Right Choice for Your Application
Selecting a material requires a clear understanding of its strengths and weaknesses in the context of your specific environment.
- If your primary focus is handling aggressive chemicals, acids, or solvents: PTFE is the benchmark material for seals, linings, and fluid-handling components due to its unparalleled inertness.
- If your application involves high-energy radiation: You must seek alternative materials, as PTFE will degrade and fail under these conditions.
- If you are working with the few known reactive agents like molten alkali metals: Be aware that you have reached the limits of PTFE's resistance and require a highly specialized material solution.
Ultimately, leveraging PTFE's power comes from respecting both its profound chemical stability and its specific operational boundaries.
Summary Table:
| Key Factor | Contribution to Chemical Resistance |
|---|---|
| Strong C-F Bonds | Extremely high bond energy makes the molecule difficult to break apart. |
| Protective Fluorine Sheath | A tight barrier of fluorine atoms shields the vulnerable carbon backbone. |
| High Electronegativity | Creates a stable, non-polar molecule that resists interaction with other substances. |
| Limitation: Radiation | PTFE is not suitable for applications involving high-energy gamma or electron beams. |
Leverage PTFE's Superior Chemical Resistance for Your Critical Components
PTFE's unparalleled inertness makes it the ideal choice for seals, liners, and labware in the semiconductor, medical, laboratory, and industrial sectors, where failure is not an option.
KINTEK specializes in the precision manufacturing of high-quality PTFE components. We understand the demanding environments your equipment faces. Whether you need standard parts or custom-fabricated solutions—from prototypes to high-volume production—we ensure reliability and performance.
Ready to enhance your system's chemical resistance? Contact our experts today to discuss your specific requirements and get a quote for components you can trust.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- Why is PTFE suitable for cryogenic or high-temperature applications? Unmatched Thermal Stability from -450°F to 500°F
- What are the primary applications of PTFE fasteners and custom parts? Critical Solutions for Extreme Environments
- What is PTFE commonly known as and what are its unique properties? Unlock Unmatched Chemical & Thermal Resistance
- What are the key benefits of PTFE in custom fabrication? Unlock Performance in Extreme Conditions
- What is the working temperature range of PTFE? Master Extreme Heat and Cryogenic Applications



















