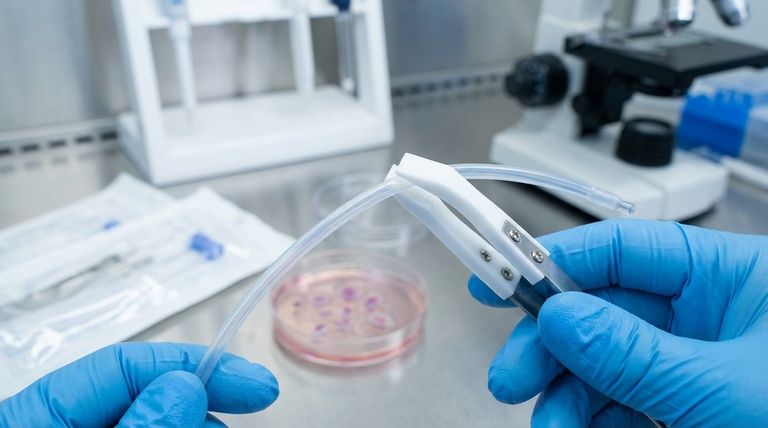
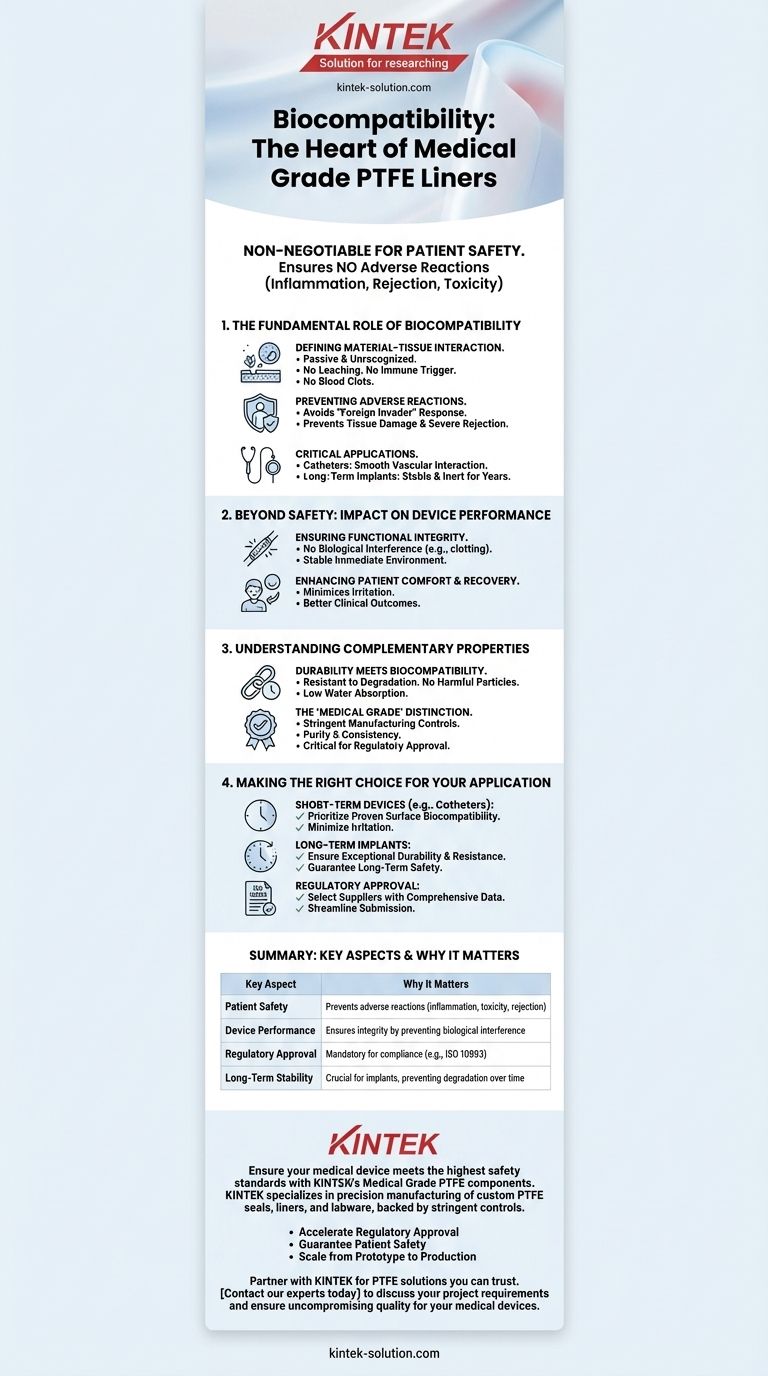
At its core, the biocompatibility of Medical Grade PTFE liners is a non-negotiable requirement for patient safety. It ensures the material will not provoke an adverse reaction—such as inflammation, rejection, or toxicity—when it comes into direct contact with bodily tissues and fluids. This inertness is fundamental to the performance and approval of critical devices like catheters and long-term implants.
The concept of biocompatibility extends beyond simply being non-toxic. It is the guarantee that a medical device will perform its intended function for a required period without causing any clinically unacceptable local or systemic responses in the patient.
The Fundamental Role of Biocompatibility
For any material used inside the human body, its interaction with the biological environment is the primary concern. PTFE's unique properties make it an excellent candidate, but only when its biocompatibility is assured.
Defining Material-Tissue Interaction
Biocompatibility is a measure of how a material coexists with a biological system. For a PTFE liner, this means it does not leach harmful substances, trigger an immune response, or cause blood clots.
It is the material's ability to remain passive and unrecognized by the body's defense mechanisms.
Preventing Adverse Bodily Reactions
A non-biocompatible material can be perceived by the body as a foreign invader. This can trigger a cascade of negative effects, from minor inflammation and discomfort to severe immune rejection or tissue damage.
Medical Grade PTFE is specifically engineered and tested to prevent these outcomes, making it a trusted material for sensitive applications.
Critical Applications: Catheters and Implants
The importance of biocompatibility is magnified in devices with intimate and prolonged tissue contact. In catheters, the liner must slide past sensitive vascular tissues without causing irritation.
For long-term implants, the material must remain stable and inert for years without degrading or causing chronic inflammation, ensuring both patient safety and device longevity.
Beyond Safety: Impact on Device Performance
While patient safety is the primary driver, biocompatibility also has a direct and significant impact on the functional performance of the medical device itself.
Ensuring Functional Integrity
An adverse tissue reaction can compromise how a device works. For example, inflammation or clotting around a catheter's tip could obstruct fluid flow or prevent accurate sensor readings.
A truly biocompatible PTFE liner ensures that the device's immediate environment remains stable, allowing it to function as designed without biological interference.
Enhancing Patient Comfort and Recovery
When a device does not provoke the body, the patient experiences greater comfort and a smoother recovery.
The inert nature of biocompatible PTFE liners minimizes post-procedural irritation and complications, contributing directly to better clinical outcomes.
Understanding the Complementary Properties
Biocompatibility does not exist in a vacuum. It must be paired with other critical material properties to be effective in a real-world medical device.
The Link Between Durability and Biocompatibility
The durability of Medical Grade PTFE is essential for maintaining its biocompatibility over time. Its resistance to chemical degradation and wear ensures that the liner does not break down into potentially harmful particles within the body.
Low water absorption also prevents the material from swelling or changing its properties, maintaining its inert state throughout the device's lifecycle.
The "Medical Grade" Distinction
The term "Medical Grade" signifies more than just the base material. It refers to a manufacturing process with stringent controls to ensure purity and eliminate contaminants that could compromise biocompatibility.
This designation provides an assurance of quality and consistency, which is critical for both regulatory approval and patient safety.
Making the Right Choice for Your Application
When selecting a PTFE liner, your decision must be guided by the specific demands and risks associated with the device's use.
- If your primary focus is short-term devices (e.g., catheters): Prioritize liners with proven surface biocompatibility to minimize tissue irritation during insertion and use.
- If your primary focus is long-term implants: Ensure the material's biocompatibility is matched by exceptional durability and resistance to degradation to guarantee long-term patient safety.
- If your primary focus is regulatory approval: Select a PTFE liner from a supplier with comprehensive biocompatibility testing data (e.g., ISO 10993) to streamline your submission process.
Ultimately, treating biocompatibility as the foundational requirement is the first step toward developing a safe, effective, and successful medical device.
Summary Table:
| Key Aspect | Why It Matters |
|---|---|
| Patient Safety | Prevents adverse reactions like inflammation, toxicity, or rejection. |
| Device Performance | Ensures functional integrity by preventing biological interference (e.g., clotting). |
| Regulatory Approval | Mandatory for compliance with standards like ISO 10993. |
| Long-Term Stability | Crucial for implants, preventing degradation and chronic inflammation over time. |
Ensure your medical device meets the highest safety standards with KINTEK's Medical Grade PTFE components.
For critical applications in the medical, semiconductor, and laboratory industries, the purity and biocompatibility of your PTFE components are non-negotiable. KINTEK specializes in the precision manufacturing of custom PTFE seals, liners, and labware, backed by the stringent controls required for medical-grade certification.
We help you:
- Accelerate Regulatory Approval: Leverage our expertise and comprehensive material data to streamline your ISO 10993 biocompatibility testing process.
- Guarantee Patient Safety: Our high-purity, inert PTFE liners are engineered to prevent adverse biological reactions in sensitive applications like catheters and long-term implants.
- Scale from Prototype to Production: Whether you need a single prototype or high-volume orders, our custom fabrication services ensure consistent quality and performance.
Partner with KINTEK for PTFE solutions you can trust. Contact our experts today to discuss your project requirements and ensure uncompromising quality for your medical devices.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Three Neck Flasks for Advanced Chemical Applications
People Also Ask
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability



















