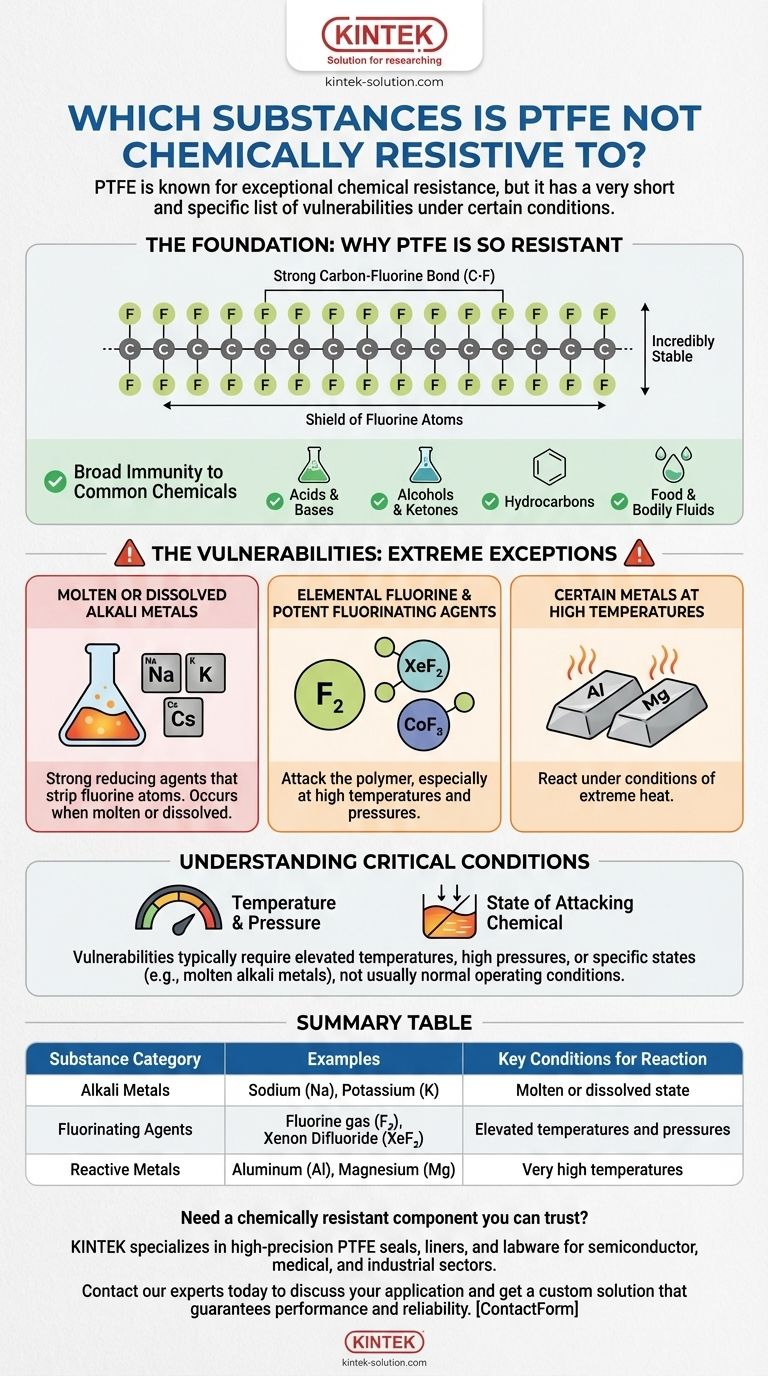
To be direct, PTFE is not chemically resistant to a very short and specific list of substances. These include molten or dissolved alkali metals like sodium, elemental fluorine gas, and certain highly reactive fluorine compounds, particularly at elevated temperatures and pressures.
While PTFE is famous for being one of the most chemically inert plastics known, its legendary stability is not absolute. Its vulnerabilities are limited to a niche class of extremely potent reducing agents and fluorinating compounds that can break its exceptionally strong carbon-fluorine bonds under specific, often extreme, conditions.

Why PTFE Is So Resistant (The Foundation)
The Strength of the Carbon-Fluorine Bond
The remarkable chemical resistance of Polytetrafluoroethylene (PTFE) stems from its molecular structure. It is composed of a long chain of carbon atoms completely shielded by a sheath of fluorine atoms.
The bond between carbon and fluorine (C-F) is one of the strongest single bonds in organic chemistry. This makes the molecule incredibly stable and non-reactive to the vast majority of chemicals.
Broad Immunity to Common Chemicals
This molecular stability gives PTFE excellent compatibility with most substances. It is unaffected by aggressive and corrosive media, including nearly all acids, alcohols, ketones, and hydrocarbons.
It remains stable when exposed to common industrial chemicals like ammonia and hydrogen peroxide, as well as food substances, enzymes, and bodily fluids, making it a premier choice for industrial, medical, and food-grade applications.
The Specific Chemical Vulnerabilities of PTFE
While the C-F bond is powerful, it is not invincible. A few highly reactive substances have the ability to attack it.
Molten or Dissolved Alkali Metals
This is the most cited exception to PTFE's chemical resistance. Alkali metals—such as sodium (Na), potassium (K), and cesium (Cs)—are extremely powerful reducing agents, especially when molten or dissolved in a solution like liquid ammonia.
These metals are aggressive enough to strip the fluorine atoms from the carbon backbone, causing the polymer to degrade.
Elemental Fluorine and Potent Fluorinating Agents
Ironically, the very element that gives PTFE its strength can also be its weakness. Elemental fluorine gas (F₂) and other rare, potent fluorinating compounds can attack the polymer, especially at high temperatures and pressures.
Examples of such reactive compounds include xenon difluoride (XeF₂) and cobalt (III) fluoride (CoF₃).
Certain Metals at High Temperatures
Under conditions of extreme heat, some other metals have been shown to react with PTFE. The most common examples cited are aluminum (Al) and magnesium (Mg) at very high temperatures.
Understanding the Critical Conditions
It is crucial to understand that these vulnerabilities are not typically present under normal operating conditions. The context of the chemical interaction is just as important as the substance itself.
The Role of Temperature and Pressure
Many of these reactions only occur at elevated temperatures and/or high pressures. At room temperature and standard pressure, PTFE may still resist some of these chemicals for a period. Its failure in these cases is conditional.
The State of the Attacking Chemical
The physical state of the substance is also critical. For instance, PTFE is vulnerable to molten or dissolved alkali metals, not solid sodium at room temperature. This distinction is vital for accurate risk assessment in an engineering context.
Making the Right Choice for Your Application
- If your primary focus is general lab or industrial use: PTFE is an exceptionally safe choice for handling nearly all common acids, bases, solvents, and petroleum products.
- If your primary focus is a high-temperature or exotic chemical environment: You must cross-reference your specific chemical agents against the known exceptions of alkali metals and potent fluorinating compounds.
- If your primary focus is a food-grade or medical application: PTFE's resistance to degradation from chemicals, enzymes, and sterilization processes makes it a highly reliable and compatible material.
Ultimately, PTFE remains one of the most chemically resistant materials available, and its well-defined limitations only apply to a narrow range of extreme substances and conditions.
Summary Table:
| Substance Category | Examples | Key Conditions for Reaction |
|---|---|---|
| Alkali Metals | Sodium (Na), Potassium (K) | Molten or dissolved state |
| Fluorinating Agents | Fluorine gas (F₂), Xenon Difluoride (XeF₂) | Elevated temperatures and pressures |
| Reactive Metals | Aluminum (Al), Magnesium (Mg) | Very high temperatures |
Need a chemically resistant component you can trust? At KINTEK, we specialize in manufacturing high-precision PTFE seals, liners, and labware for the semiconductor, medical, and industrial sectors. Our custom fabrication ensures your components are perfectly suited to handle your specific chemical environment, avoiding the rare vulnerabilities of PTFE.
Contact our experts today to discuss your application and get a custom solution that guarantees performance and reliability.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
People Also Ask
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss
- In which industries is PTFE commonly used? Key Applications for Chemical & Thermal Resistance
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance



















