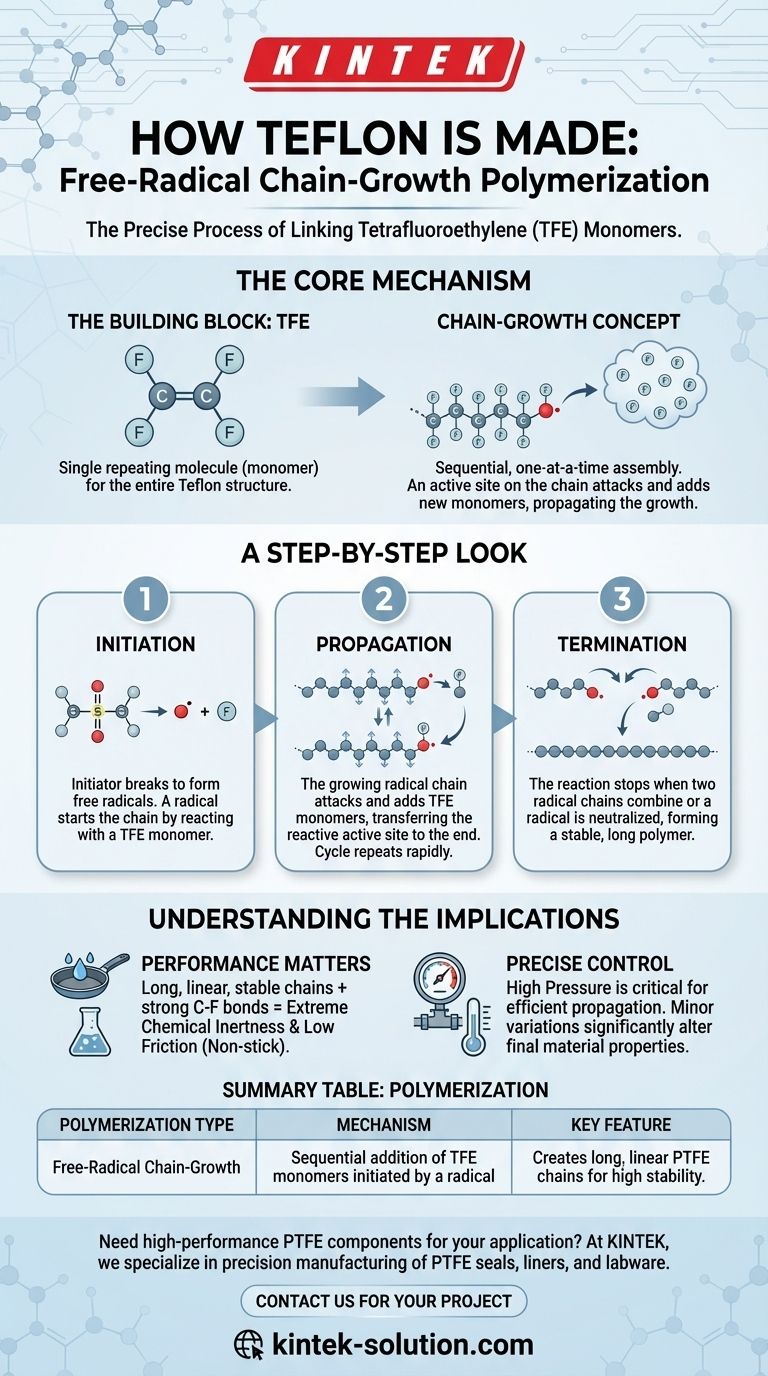
To be precise, Teflon is created through a specific type of polymerization called free-radical chain-growth polymerization. This process involves linking individual molecules of a gas called tetrafluoroethylene (TFE) together into long, stable chains under high pressure with the help of a persulphate initiator.
The key to understanding how Teflon is made is to see it not as a simple mixing of ingredients, but as a rapid, self-sustaining chain reaction where a single active molecule triggers a cascade, adding one building block at a time to create the final polymer.

The Core Mechanism: Chain-Growth Polymerization
To grasp how Teflon is formed, we must first understand its fundamental building block and the nature of a "chain-growth" reaction. This method is defined by its sequential, one-at-a-time assembly process.
The Building Block: Tetrafluoroethylene (TFE)
The entire structure of Teflon (chemically known as Polytetrafluoroethylene or PTFE) comes from a single, repeating molecule: tetrafluoroethylene (TFE).
You can think of TFE as the individual link in what will become a very long chain.
The "Chain-Growth" Concept
In chain-growth polymerization, an "active site" is created on one end of a monomer. This active site then attacks another monomer, adds it to the chain, and transfers the active site to the newly added end.
This process repeats thousands of times, rapidly growing the polymer chain one link at a time. It is distinct from other methods where large polymer fragments might combine.
A Step-by-Step Look at the Free-Radical Process
The "free-radical" part of the name describes how the reaction gets started and keeps going. The entire process can be broken down into three distinct phases.
Step 1: Initiation
The reaction doesn't start on its own. It requires an initiator (the references call this a persulphate catalyst) to kick things off.
This initiator molecule breaks apart to form free radicals—highly unstable molecules with an unpaired electron. This radical immediately seeks to stabilize itself by reacting with a stable TFE monomer, officially starting the polymer chain.
Step 2: Propagation
This is the heart of the reaction. The initial TFE monomer, having reacted with the free radical, is now itself a radical.
This new, larger radical attacks another TFE monomer, adding it to the chain and passing the reactive "hot potato" of the free radical to the very end of the growing chain. This cycle repeats extremely quickly, propagating the chain's growth.
Step 3: Termination
The chain reaction cannot continue forever. It eventually stops, or terminates, when two growing radical chains meet and combine, or when the radical is neutralized by another molecule.
The length of the final polymer chains, which dictates the material's properties, is determined by how long propagation continues before termination occurs.
Understanding the Implications
The choice of this polymerization method is not arbitrary; it is directly responsible for the unique and valuable properties of Teflon.
Why This Process Matters for Performance
Free-radical chain-growth polymerization creates very long, linear, and stable polymer chains.
The resulting structure, combined with the immense strength of the carbon-fluorine bond in each TFE unit, is what gives Teflon its signature properties: extreme chemical inertness and a very low coefficient of friction (its "non-stick" quality).
The Need for Precise Control
The conditions mentioned in the references—specifically high pressure—are critical. High pressure keeps the gaseous TFE monomer concentrated, ensuring the growing radical chain can find its next link efficiently and continue the propagation step.
Minor variations in pressure, temperature, or initiator concentration can significantly alter the final polymer, affecting its molecular weight and performance characteristics.
Making the Right Choice for Your Goal
Understanding this process allows you to connect the molecular assembly to the final material properties.
- If your primary focus is chemistry: The key takeaway is that Teflon is a classic example of addition polymerization, where a free-radical initiator creates a self-sustaining reaction with TFE monomers.
- If your primary focus is materials science: The takeaway is that the chain-growth method produces the high-molecular-weight, linear PTFE structure required for exceptional thermal stability and chemical resistance.
- If your primary focus is practical application: The takeaway is that this controlled chain reaction is what builds the incredibly stable, non-reactive surface that makes everyday non-stick cookware possible.
Ultimately, comprehending the polymerization process reveals how we can assemble simple molecules into materials with extraordinary capabilities.
Summary Table:
| Polymerization Type | Mechanism | Key Feature |
|---|---|---|
| Free-Radical Chain-Growth | Sequential addition of TFE monomers initiated by a radical | Creates long, linear PTFE chains for high stability |
Need high-performance PTFE components for your application? At KINTEK, we specialize in precision manufacturing of PTFE seals, liners, and labware for semiconductor, medical, and industrial sectors. Our custom fabrication—from prototypes to high-volume orders—ensures material integrity by understanding the precise polymerization process behind PTFE. Contact us today to discuss your project and leverage our expertise in advanced polymer solutions!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Rods for Advanced Industrial Applications
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
People Also Ask
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- What are the key advantages of PTFE? Unmatched Performance for Extreme Environments
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs



















