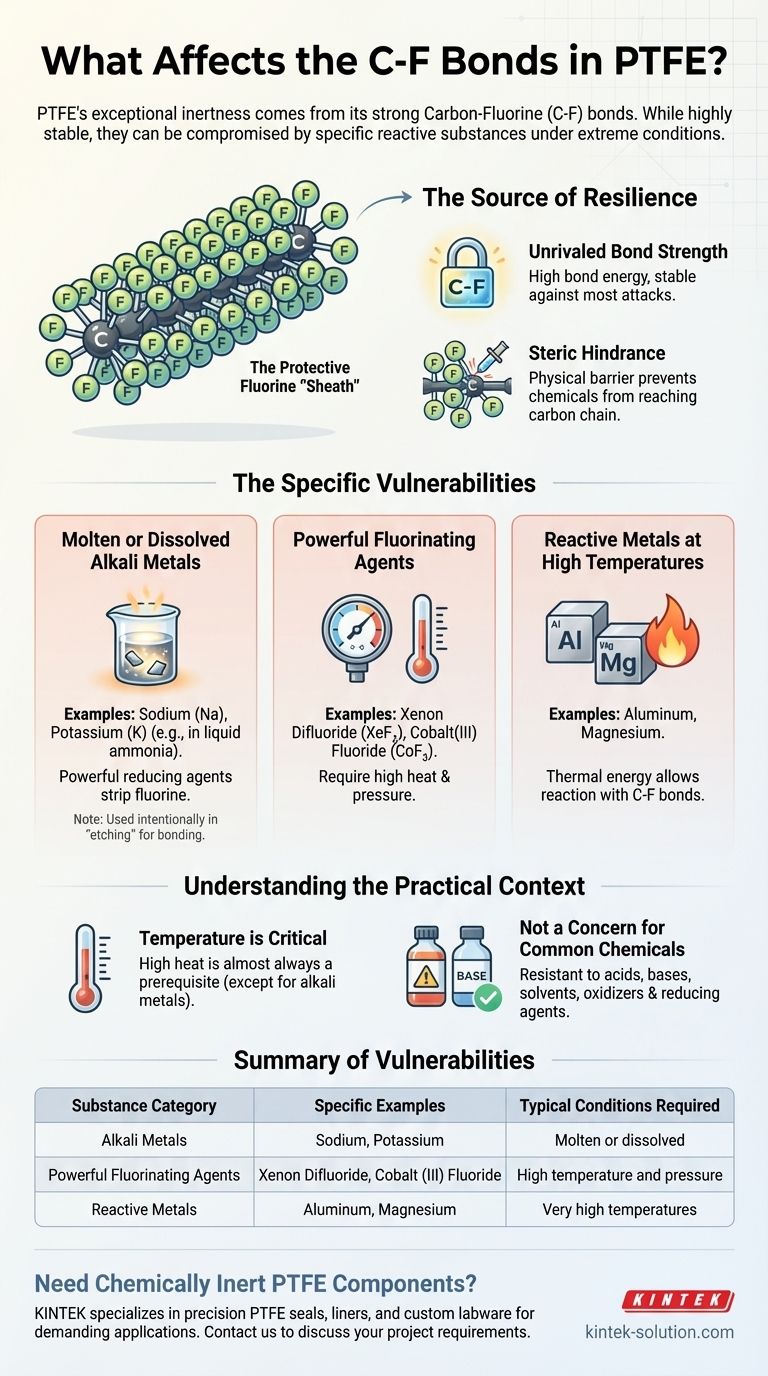
While famously inert, the carbon-fluorine (C-F) bonds in Polytetrafluoroethylene (PTFE) are not invincible. A small, specific group of highly reactive substances can affect them, but typically only under extreme conditions. These include molten or dissolved alkali metals, certain powerful fluorinating agents at high temperatures and pressures, and specific reactive metals like aluminum or magnesium at high temperatures.
The exceptional strength and stability of the carbon-fluorine bond is the source of PTFE's legendary chemical resistance. Only the most aggressive chemical agents, often combined with high energy input like extreme heat, possess the necessary reactivity to overcome this bond and degrade the material.

The Source of PTFE's Resilience
The chemical inertness of PTFE is not an accident; it is a direct result of its molecular structure. Understanding this structure is key to understanding its few vulnerabilities.
The Unrivaled Strength of the C-F Bond
The bond between a carbon atom and a fluorine atom is one of the strongest known single bonds in organic chemistry. This high bond energy means a significant amount of energy is required to break it, making it stable against most chemical attacks.
A Protective Fluorine "Sheath"
In the PTFE polymer, the carbon backbone is completely encased by a dense, helical sheath of fluorine atoms. This physical barrier sterically hinders other chemicals from even reaching the carbon chain, effectively shielding it from attack.
The Specific Substances That Can Affect PTFE
Only a few materials are reactive enough to overcome PTFE's defenses. These scenarios are rare and typically fall outside of normal industrial or commercial use.
Molten or Dissolved Alkali Metals
This is the most well-known vulnerability of PTFE. Alkali metals like sodium and potassium, particularly when molten or dissolved in solutions like liquid ammonia, are extremely powerful reducing agents. They can aggressively strip fluorine atoms from the polymer backbone.
This very reaction is leveraged intentionally in a process called etching, which breaks the C-F bonds on the surface to make the otherwise non-stick PTFE bondable with adhesives.
Powerful Fluorinating Agents
Under conditions of high heat and pressure, exotic and highly aggressive fluorinating compounds can affect PTFE. Examples include xenon difluoride and cobalt (III) fluoride. These are not substances encountered in typical chemical processing.
Reactive Metals at High Temperatures
Certain reactive metals, specifically aluminum and magnesium, can react with PTFE at very high temperatures. The thermal energy overcomes the activation barrier, allowing these metals to interact with and break the C-F bonds.
Understanding the Practical Context
It is critical to frame these vulnerabilities correctly. For the vast majority of applications, they are theoretical limits rather than practical concerns.
Temperature is a Critical Factor
With the exception of alkali metals, high temperature is almost always a prerequisite for a chemical reaction with PTFE. In most cases, you would reach PTFE's own thermal degradation limits before these specific chemical reactions become a concern.
Not a Concern for Common Chemicals
PTFE is completely resistant to virtually all common industrial chemicals. This includes strong acids, bases, solvents, oxidizers, and reducing agents you are likely to encounter in a lab or plant.
Etching as a Controlled Application
The reaction with alkali metals is a prime example of turning a vulnerability into a tool. Sodium-based etchants are the standard industry method for preparing a PTFE surface for gluing or potting, a task that would otherwise be impossible.
Assessing PTFE for Your Application
Your final decision should be based on a realistic assessment of the operational environment.
- If your primary focus is general chemical resistance: PTFE remains one of the most reliable and inert materials available for sealing, lining, and fluid handling.
- If you need to bond PTFE to another surface: You will need to employ a chemical etching process using an alkali metal formulation to break the surface C-F bonds.
- If your application involves molten sodium, high-temperature aluminum, or exotic fluorinating agents: PTFE is unsuitable, and you must select a different material for chemical compatibility.
Understanding these niche limitations allows you to confidently leverage PTFE's exceptional inertness across the vast majority of demanding applications.
Summary Table:
| Substance Category | Specific Examples | Typical Conditions Required |
|---|---|---|
| Alkali Metals | Sodium, Potassium | Molten or dissolved (e.g., in liquid ammonia) |
| Powerful Fluorinating Agents | Xenon Difluoride, Cobalt (III) Fluoride | High temperature and pressure |
| Reactive Metals | Aluminum, Magnesium | Very high temperatures |
Need Chemically Inert PTFE Components for Your Application?
PTFE's legendary chemical resistance makes it the ideal choice for sealing, lining, and fluid handling in the most demanding environments. However, selecting the right material for your specific conditions is critical.
KINTEK specializes in the precision manufacturing of high-performance PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. We can help you navigate material limitations to ensure optimal performance and longevity.
Let us provide the solution for your specialized needs:
- Custom Fabrication: From prototypes to high-volume production runs.
- Expert Guidance: Leverage our deep material science knowledge.
- Precision Production: Ensure reliability in your critical processes.
Contact KINTEK today to discuss your project requirements and discover how our PTFE expertise can benefit you.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss
- What industrial applications does PTFE have? Unlock Performance in Extreme Environments
- In which industries is PTFE commonly used? Key Applications for Chemical & Thermal Resistance
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments



















