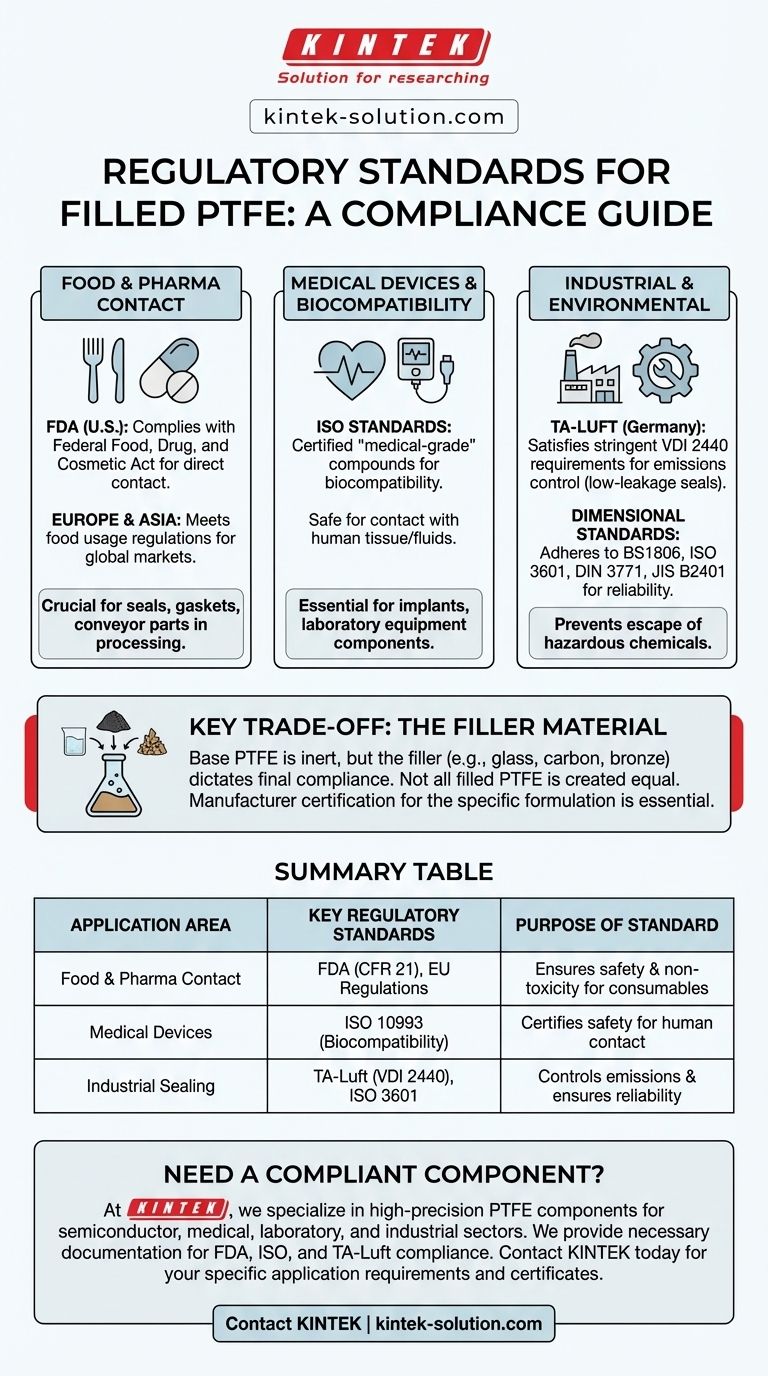
In short, filled PTFE complies with a range of stringent international standards. This includes regulations for food contact, medical devices, and industrial emissions, making it a highly versatile material for critical applications. Key standards it often meets include those set by the FDA, ISO, and Germany's TA-Luft for leakage control.
The specific regulatory compliance of a filled PTFE product depends entirely on its final formulation. While the base PTFE resin is often compliant, the type of filler used dictates its suitability for applications like food processing, medical implants, or industrial sealing.

Food and Drug Contact Regulations
When a material is used in food processing, pharmaceutical manufacturing, or medical devices, its safety and non-reactivity are paramount. Filled PTFE compounds are frequently certified to meet these demanding requirements.
The FDA Standard (U.S.)
Many filled PTFE formulations conform to the Federal Food, Drug, and Cosmetic Act. This certification from the U.S. Food and Drug Administration (FDA) confirms the material is safe for direct contact with food and pharmaceutical products.
This makes it a standard choice for components like seals, gaskets, and conveyor parts in processing equipment.
European and Asian Regulations
Beyond the U.S., filled PTFE also meets food usage regulations in Europe and Asia. This broad compliance ensures that products manufactured with these materials can be deployed in global markets without facing regulatory hurdles.
Biocompatibility for Medical Use
For medical applications, specific "medical-grade" PTFE compounds comply with ISO (International Organization for Standardization) standards. These certifications ensure the material is biocompatible, meaning it will not cause adverse reactions when in contact with the human body.
This is critical for components used in medical devices and laboratory equipment.
Industrial and Environmental Standards
In demanding industrial environments like chemical and petrochemical plants, regulations focus on safety, reliability, and environmental protection.
TA-Luft for Emissions Control
Filled PTFE gaskets and seals often satisfy the TA-Luft 2002 [VDI 2440/2200] requirements. This is a rigorous German technical instruction on air quality control that sets strict limits for fugitive emissions from industrial flange connections.
Meeting this standard signifies that the material provides a superior, low-leakage seal, which is essential for preventing the escape of hazardous chemicals.
Dimensional and Mechanical Standards
For standardized shapes like O-rings, PTFE also adheres to mechanical and dimensional standards such as BS1806, ISO 3601, DIN 3771, and JIS B2401. While these relate more to physical specifications than material composition, they ensure interchangeability and reliable performance in standard equipment.
Understanding the Key Trade-off: The Filler Material
The most critical factor in regulatory compliance is the composition of the final material. Not all filled PTFE is created equal, and assuming compliance can be a costly mistake.
The Impact of the Filler
The base PTFE polymer is inherently inert and non-toxic. However, fillers like glass, carbon, bronze, or stainless steel are added to enhance mechanical properties like wear resistance or compressive strength.
The filler material itself must also be approved for the intended application. For example, a PTFE compound with a non-food-grade filler cannot be used in a food processing line, even if the base PTFE is compliant.
The Necessity of Manufacturer Certification
Compliance is not inherent to the material category; it is certified for a specific formulation. You must always obtain a certificate of compliance from the manufacturer that explicitly states the standards met by that particular product.
This documentation is your proof that the material is appropriate and safe for your specific use case.
Making the Right Choice for Your Application
To ensure you select the correct material, your specification must be driven by your application's regulatory environment.
- If your primary focus is food or pharmaceutical processing: Specify a PTFE compound that is explicitly certified to FDA and/or relevant EU food contact standards.
- If your primary focus is medical devices: You must source a medical-grade PTFE that is certified for biocompatibility under the appropriate ISO standards.
- If your primary focus is industrial sealing in chemical plants: Look for materials that have been tested and certified to meet TA-Luft leakage requirements.
Always verify the material's specific certifications with the supplier to guarantee it meets the precise demands of your project.
Summary Table:
| Application Area | Key Regulatory Standards | Purpose of Standard |
|---|---|---|
| Food & Pharma Contact | FDA (CFR 21), EU Food Contact Regulations | Ensures material safety and non-toxicity for consumable products |
| Medical Devices & Biocompatibility | ISO 10993 (Biocompatibility) | Certifies safety for contact with human tissues/fluids |
| Industrial Sealing & Emissions | TA-Luft (VDI 2440), BS1806, ISO 3601 | Controls fugitive emissions and ensures mechanical reliability |
Need a filled PTFE component that meets strict regulatory demands?
At KINTEK, we specialize in manufacturing high-precision PTFE components (seals, liners, labware, and more) for the semiconductor, medical, laboratory, and industrial sectors. We understand that compliance is not optional—it's critical. Whether you require FDA-compliant seals for a food processing line, ISO-certified components for a medical device, or TA-Luft validated gaskets for chemical plant safety, we provide the necessary documentation and material traceability.
We offer custom fabrication from prototypes to high-volume orders, ensuring every part meets your exact specifications and regulatory requirements.
Contact KINTEK today to discuss your project and receive a certificate of compliance for your specific application.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
People Also Ask
- What are some common applications for PTFE rotary seals? Sealing Solutions for Extreme Environments
- What are PTFE balls made of and what are their key properties? Unlock Superior Chemical & Thermal Performance
- What other applications benefit from PTFE lubrication? Solve Extreme Lubrication & Sealing Challenges
- What are some specialized industrial applications of guide rings? Essential Uses for Oil-Free and High-Load Systems
- What modifications exist for PTFE O-ring temperature performance? Enhance High-Temp Stability with Fillers
- What is the expected service life of expanded PTFE gaskets? Maximize Sealing Longevity in Harsh Environments
- What are the key characteristics of PTFE Enveloped Gaskets? Solve Demanding Sealing Challenges
- What are the benefits of CNC machining for PTFE parts? Achieve Precision and Performance



















