The short answer is that Teflon's remarkable chemical resistance is due to the immense strength and stability of the carbon-fluorine bonds that make up its molecular structure. These bonds create an incredibly non-reactive surface, effectively shielding the molecule's carbon backbone from attack by nearly all acids, bases, and solvents.
The core reason for Teflon's inertness lies in its atomic architecture. A protective sheath of fluorine atoms, bonded with exceptional strength to a carbon spine, leaves no easy entry point for other chemicals to initiate a reaction.

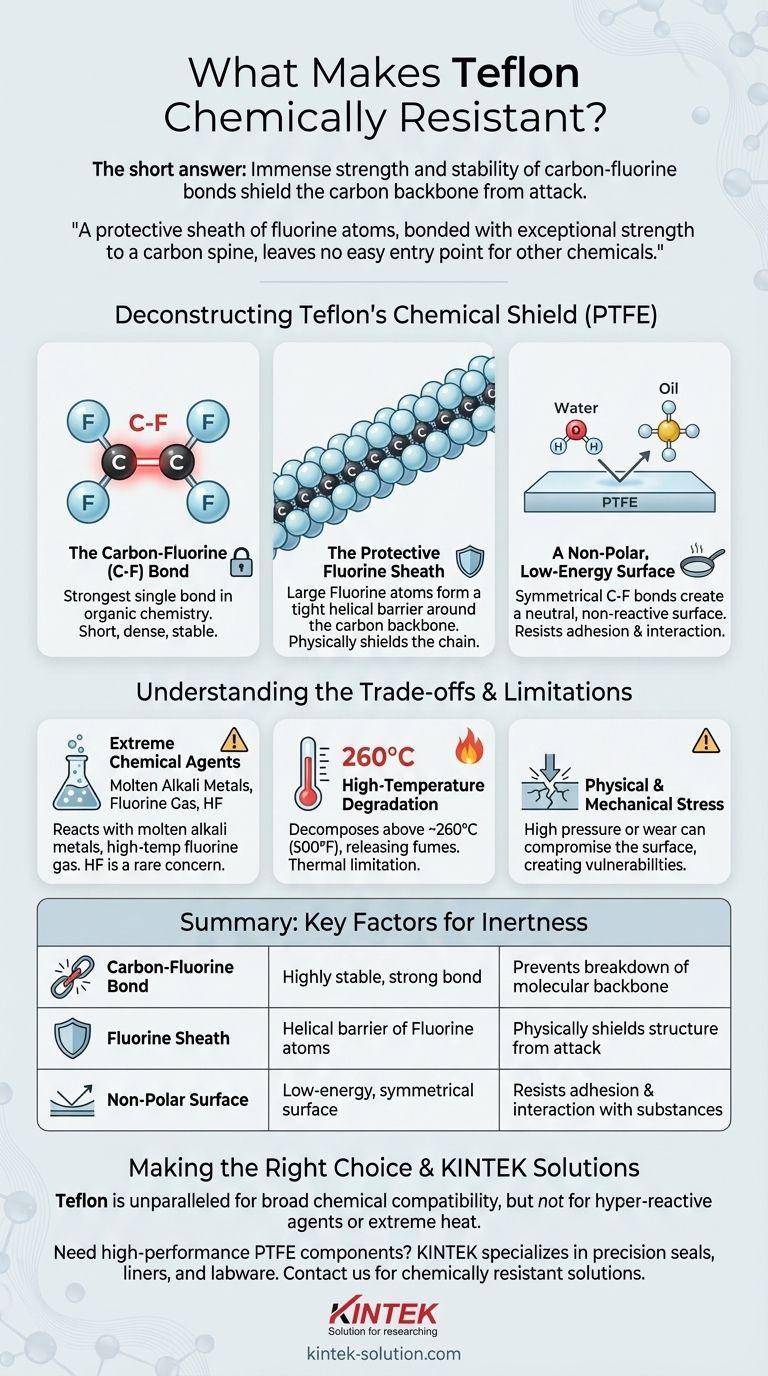
Deconstructing Teflon's Chemical Shield
To understand Teflon's resistance, we must look at its structure at the molecular level. Teflon is the brand name for a polymer, most commonly Polytetrafluoroethylene (PTFE).
The Carbon-Fluorine Bond
The defining feature of PTFE is the carbon-fluorine (C-F) bond. This bond is one of the strongest single bonds in all of organic chemistry.
Fluorine is the most electronegative element, meaning it has an extremely strong pull on electrons. This creates a very short, dense, and stable bond with carbon that is difficult to break.
The Protective Fluorine Sheath
A PTFE molecule consists of a long chain of carbon atoms. Each carbon atom is bonded to two fluorine atoms.
These fluorine atoms are larger than the carbon atoms they are attached to, causing them to form a tight, helical sheath around the carbon backbone. This physical barrier further protects the more vulnerable carbon chain from chemical attack.
A Non-Polar, Low-Energy Surface
The symmetrical arrangement of the C-F bonds results in a non-polar surface. This lack of electrical charge means it has very weak interactions with other molecules, including polar solvents like water or non-polar solvents like oils.
This low surface energy is also why Teflon is known for its non-stick properties; other substances have nothing to "grab" onto.
Understanding the Trade-offs and Limitations
While extraordinarily resistant, Teflon is not invincible. Understanding its few weaknesses is critical for safe and effective application.
Extreme Chemical Agents
A handful of highly reactive substances can attack Teflon under specific conditions. These include molten alkali metals like sodium and potent fluorinating agents such as elemental fluorine gas at high temperatures and pressures.
Some sources also note that hydrofluoric acid (HF) can be a concern for certain Teflon-encapsulated components, a rare exception to its acid resistance.
High-Temperature Degradation
Teflon's chemical resistance is tied to its physical integrity. At very high temperatures (generally above 260°C or 500°F for PTFE), the material will begin to break down.
Direct flame will cause it to decompose, releasing potentially hazardous fumes. This is a thermal limitation, not a typical chemical reactivity issue.
Physical and Mechanical Stress
In harsh environments, physical stress can create vulnerabilities. High pressure or mechanical wear can compromise the material's surface, potentially creating sites where aggressive chemicals could begin to degrade the polymer.
Making the Right Choice for Your Application
Selecting a material requires matching its properties to the operational environment.
- If your primary focus is broad chemical compatibility: Teflon is an unparalleled choice for handling the vast majority of common and aggressive acids, bases, and organic solvents in lab or industrial settings.
- If your primary focus is high-temperature processing: You must verify the specific temperature rating of the Teflon variant (e.g., PTFE, PFA, FEP) and ensure it remains well below its decomposition point.
- If your primary focus involves niche, hyper-reactive chemicals: For applications involving molten alkali metals or high-pressure fluorine gas, Teflon is not a suitable material and alternatives must be found.
Ultimately, Teflon's chemical inertness is a direct result of its unique and robust molecular design.
Summary Table:
| Key Factor | Explanation | Impact on Chemical Resistance |
|---|---|---|
| Carbon-Fluorine Bond | One of the strongest bonds in organic chemistry, highly stable. | Prevents most chemical reactions from breaking the molecular backbone. |
| Fluorine Sheath | Fluorine atoms form a protective helical barrier around the carbon chain. | Physically shields the structure from chemical attack. |
| Non-Polar Surface | Symmetrical C-F bonds create a low-energy, non-reactive surface. | Resists adhesion and interaction with polar and non-polar substances. |
Need high-performance PTFE components for your demanding applications? At KINTEK, we specialize in manufacturing precision PTFE seals, liners, and labware for the semiconductor, medical, laboratory, and industrial sectors. Our custom fabrication services—from prototypes to high-volume orders—ensure you get chemically resistant solutions tailored to your exact needs. Contact us today to discuss how our PTFE expertise can enhance your process reliability and safety!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
People Also Ask
- What is the working temperature range of PTFE? Master Extreme Heat and Cryogenic Applications
- Why is PTFE suitable for cryogenic or high-temperature applications? Unmatched Thermal Stability from -450°F to 500°F
- What are the primary applications of PTFE fasteners and custom parts? Critical Solutions for Extreme Environments
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications
- What are the key benefits of PTFE in custom fabrication? Unlock Performance in Extreme Conditions



















