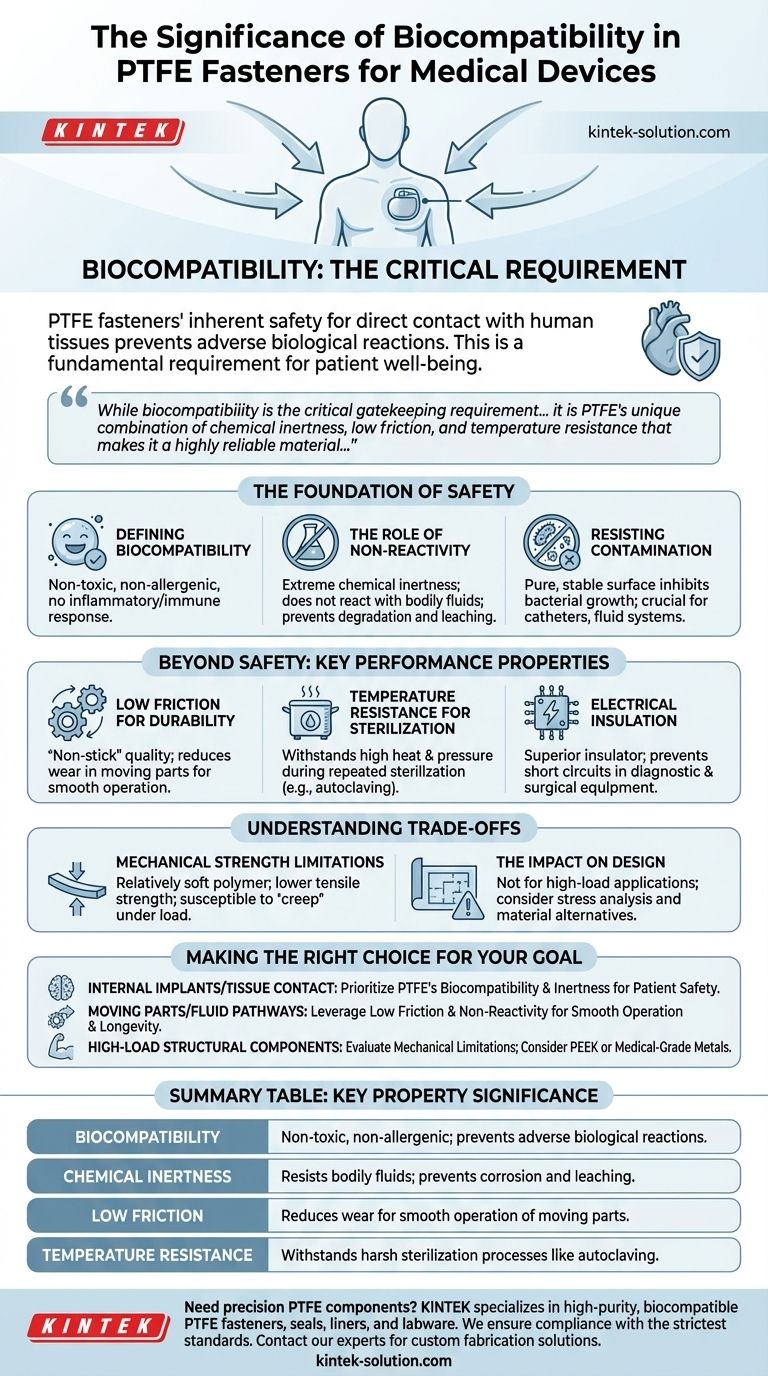
The significance of biocompatibility in PTFE fasteners is their inherent safety for direct contact with human tissues, preventing adverse biological reactions. This property is not just a benefit but a fundamental requirement for any component used in medical implants or devices that have prolonged interaction with the body, ensuring patient well-being is never compromised.
While biocompatibility is the critical gatekeeping requirement for patient safety, it is PTFE's unique combination of chemical inertness, low friction, and temperature resistance that makes it a highly reliable material for ensuring the long-term integrity and performance of critical medical devices.
The Foundation of Safety: Biocompatibility and Chemical Inertness
The primary reason PTFE is specified for medical applications is its relationship with living tissue. This is a direct result of its fundamental chemical structure.
Defining Biocompatibility
Biocompatibility means a material is non-toxic, non-allergenic, and does not trigger an inflammatory or immune response when introduced to the body. For a fastener, this ensures it can perform its mechanical function without causing harm to the surrounding tissues.
The Role of Non-Reactivity
PTFE’s biocompatibility stems from its extreme chemical inertness. It does not react with bodily fluids, acids, or bases, meaning the fastener will not degrade, corrode, or leach harmful substances over time.
This non-reactivity is crucial for maintaining the integrity of the device and preventing contamination within the patient's body or in sensitive laboratory equipment.
Resisting Contamination
The material's purity and stable chemical nature also make its surface less hospitable to bacterial growth. This is vital in applications like catheters or fluid handling systems, where minimizing the risk of infection is paramount.
Beyond Safety: Key Performance Properties
While safety is the prerequisite, PTFE offers a suite of other properties that enhance the function and reliability of medical devices.
Low Friction for Durability
PTFE has one of the lowest coefficients of friction of any solid material. This "non-stick" quality is ideal for fasteners in devices with moving parts, as it reduces wear and tear and ensures smooth, reliable operation over the device's lifespan.
Temperature Resistance for Sterilization
Medical instruments often undergo harsh sterilization processes, such as autoclaving, which involves high heat and pressure. PTFE's ability to withstand a wide range of temperatures ensures that fasteners maintain their structural integrity and performance after repeated sterilization cycles.
Electrical Insulation
As a superior electrical insulator, PTFE is also valuable in complex diagnostic and surgical equipment. Fasteners made from PTFE can prevent electrical shorts and ensure the safe operation of electronic components within the device.
Understanding the Trade-offs
No material is perfect for every application. Acknowledging the limitations of PTFE is essential for sound engineering and device design.
Mechanical Strength Limitations
PTFE is a relatively soft polymer compared to high-performance plastics like PEEK or medical-grade metals. It has lower tensile strength and is more susceptible to "creep," which is slow deformation under a constant load.
The Impact on Design
This means PTFE fasteners are not suitable for high-load or structurally demanding applications. Engineers must carefully analyze the mechanical stresses on a component before specifying PTFE, reserving it for applications where its unique chemical and surface properties are the primary requirements.
Making the Right Choice for Your Goal
Selecting the correct material requires balancing safety requirements with mechanical demands.
- If your primary focus is internal implants or long-term tissue contact: PTFE's proven biocompatibility and chemical inertness make it an essential choice for ensuring patient safety.
- If your primary focus is devices with moving parts or fluid pathways: The combination of low friction and non-reactivity ensures smooth operation, longevity, and prevents contamination.
- If your primary focus is high-load structural components: You must evaluate the mechanical limitations of PTFE and consider alternative materials like PEEK or medical-grade metals.
Ultimately, leveraging PTFE's unique properties correctly allows for the creation of safer, more reliable, and longer-lasting medical devices.
Summary Table:
| Property | Significance for Medical Devices |
|---|---|
| Biocompatibility | Non-toxic, non-allergenic; prevents adverse biological reactions. |
| Chemical Inertness | Resists bodily fluids; prevents corrosion and leaching. |
| Low Friction | Reduces wear for smooth operation of moving parts. |
| Temperature Resistance | Withstands harsh sterilization processes like autoclaving. |
Need precision PTFE components for your critical medical, semiconductor, or laboratory applications? KINTEK specializes in manufacturing high-purity, biocompatible PTFE fasteners, seals, liners, and labware. We ensure your components meet the strictest safety and performance standards, offering custom fabrication from prototypes to high-volume orders. Contact our experts today to discuss how we can support your project with reliable, precision-engineered solutions.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications



















