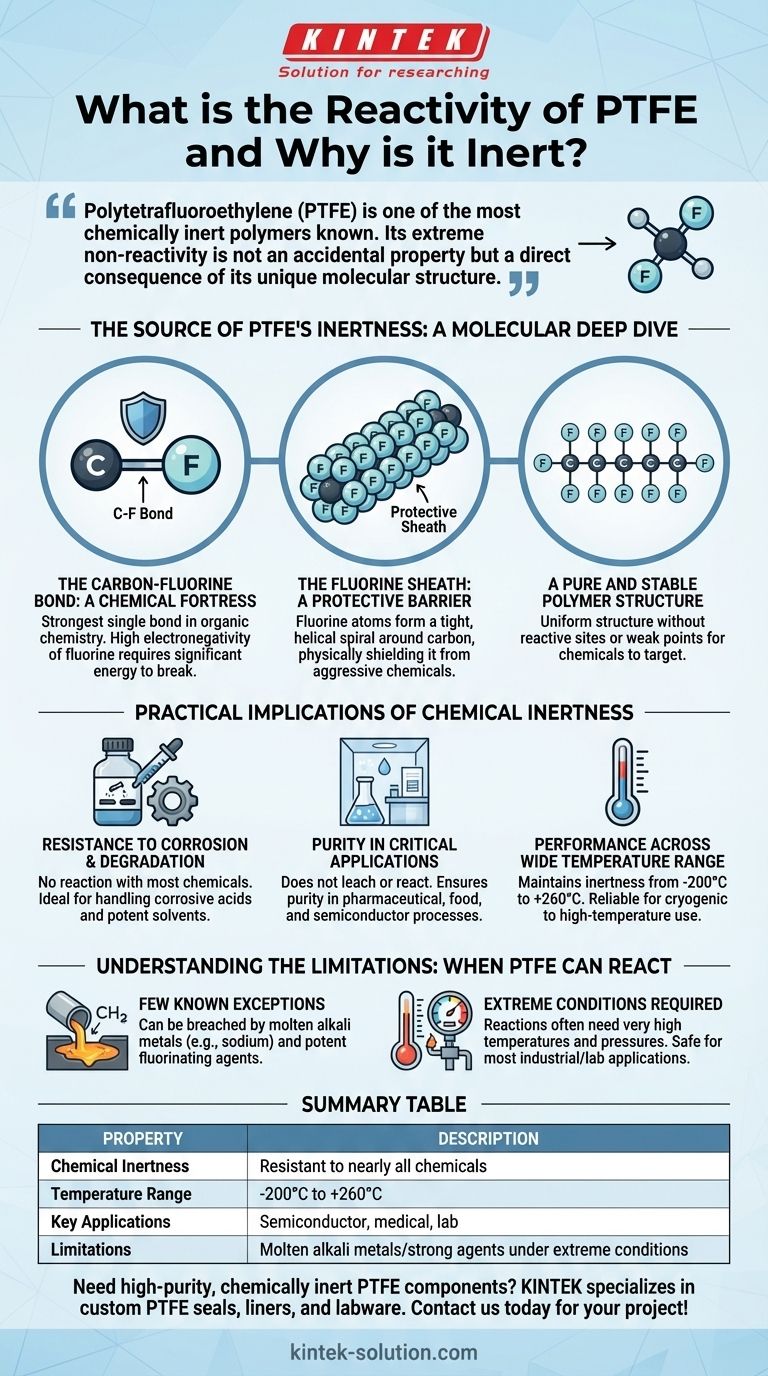
Polytetrafluoroethylene (PTFE) is one of the most chemically inert polymers known. Its extreme non-reactivity is not an accidental property but a direct consequence of its unique molecular structure. The exceptionally strong bonds between its carbon and fluorine atoms create a stable, protected molecule that is virtually impervious to chemical attack from acids, alkalis, and solvents.
The source of PTFE's profound chemical inertness lies in its molecular architecture. A protective sheath of tightly-packed fluorine atoms shields a stable carbon backbone, creating a formidable barrier that prevents interaction with nearly all external substances.

The Source of PTFE's Inertness: A Molecular Deep Dive
To understand why PTFE is so unreactive, we must look at its structure on a chemical level. The properties emerge from a combination of bond strength and physical shielding.
The Carbon-Fluorine Bond: A Chemical Fortress
The fundamental building block of PTFE is the carbon-fluorine (C-F) bond. This is one of the strongest single bonds in organic chemistry.
This strength arises from the high electronegativity of fluorine atoms, which form a very stable and low-energy bond with carbon, requiring a significant amount of energy to break.
The Fluorine Sheath: A Protective Barrier
The PTFE molecule consists of a long chain of carbon atoms, but this "backbone" is not exposed. It is completely surrounded by fluorine atoms.
Because fluorine atoms are larger than carbon atoms, they arrange themselves in a tight, helical spiral around the carbon chain. This dense outer layer of fluorine acts as a physical shield, preventing aggressive chemicals from ever reaching the more vulnerable carbon backbone.
A Pure and Stable Polymer Structure
PTFE's structure is simple and uniform, consisting only of carbon and fluorine atoms. It lacks the reactive sites, such as double bonds or hydrogen atoms, that make other polymers susceptible to chemical attack.
This purity means there are no weak points in the molecular chain for chemicals to target, further contributing to its overall stability.
Practical Implications of Chemical Inertness
This molecular stability translates directly into valuable real-world properties, making PTFE a critical material in demanding industries.
Resistance to Corrosion and Degradation
Because PTFE does not react with most chemicals, it does not corrode, rust, or degrade when exposed to them.
This makes it an ideal material for handling almost any liquid or gas—from highly corrosive acids to potent solvents—without breaking down.
Purity in Critical Applications
In environments like pharmaceutical labs, food processing, or semiconductor manufacturing, even minute contamination can be disastrous.
PTFE's inertness ensures it will not leach chemicals or react with the substances it contains, guaranteeing the purity of the end product.
Performance Across a Wide Temperature Range
PTFE maintains its chemical inertness and structural integrity across an exceptionally broad temperature range, typically from –200° C to +260° C.
This allows it to be used reliably in applications involving both cryogenic fluids and high-temperature chemical processing.
Understanding the Limitations: When PTFE Can React
While PTFE is practically inert, it is not completely immune to attack under all conceivable conditions. Understanding its few limitations is critical for safe and effective use.
The Few Known Exceptions
PTFE's chemical fortress can be breached by a very small number of highly reactive substances.
These exceptions primarily include molten alkali metals (like sodium), and potent fluorinating agents such as chlorine trifluoride and elemental fluorine itself.
The Role of Extreme Conditions
It is crucial to note that these reactions often require extreme conditions, such as very high temperatures and pressures, to occur.
For the vast majority of industrial and laboratory applications, PTFE remains completely non-reactive and reliable.
Making the Right Choice for Your Application
Selecting a material requires matching its properties to your goal. PTFE's inertness makes it a premier choice for applications where chemical resistance is paramount.
- If your primary focus is handling aggressive chemicals: PTFE is an industry standard, offering unparalleled resistance to nearly all acids, bases, and solvents.
- If your primary focus is maintaining product purity: PTFE's non-reactive nature ensures it will not contaminate sensitive processes, making it ideal for food, pharmaceutical, or laboratory use.
- If your primary focus is performance in extreme environments: You must verify that your application does not involve PTFE's few chemical exceptions, particularly molten alkali metals or specific fluorine compounds at high temperatures.
Ultimately, understanding the molecular basis of PTFE's stability allows you to deploy it with confidence in the most demanding chemical environments.
Summary Table:
| Property | Description |
|---|---|
| Chemical Inertness | Resistant to nearly all acids, bases, and solvents due to strong C-F bonds and fluorine shielding. |
| Temperature Range | Maintains stability from -200°C to +260°C. |
| Key Applications | Ideal for semiconductor, medical, pharmaceutical, and laboratory use where purity is critical. |
| Limitations | Can react with molten alkali metals and strong fluorinating agents under extreme conditions. |
Need high-purity, chemically inert PTFE components for your critical applications? At KINTEK, we specialize in manufacturing custom PTFE seals, liners, and labware for the semiconductor, medical, and industrial sectors. Our precision production ensures reliability in the most demanding environments. Contact us today to discuss your project requirements—from prototypes to high-volume orders!
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- Why is PTFE considered safe for food and beverage applications? Ensuring Product Purity and Operational Safety
- What are the common applications of PTFE? Leverage Its Extreme Properties for Your Industry
- What makes PTFE's low friction properties advantageous for aerospace applications? Achieve Unmatched Reliability and Efficiency
- What does PTFE stand for and what are its primary characteristics? | The Ultimate High-Performance Polymer
- Why is PTFE considered an ideal substitute for other plastics in high-temperature applications? Superior Thermal Stability & Performance
- What is PTFE and what are its basic characteristics? Discover the Power of a High-Performance Polymer
- What is the chemical composition of PTFE? Unlocking the Power of Carbon-Fluorine Bonds
- What is PTFE commonly used for in construction? Essential Applications for High-Performance Infrastructure



















