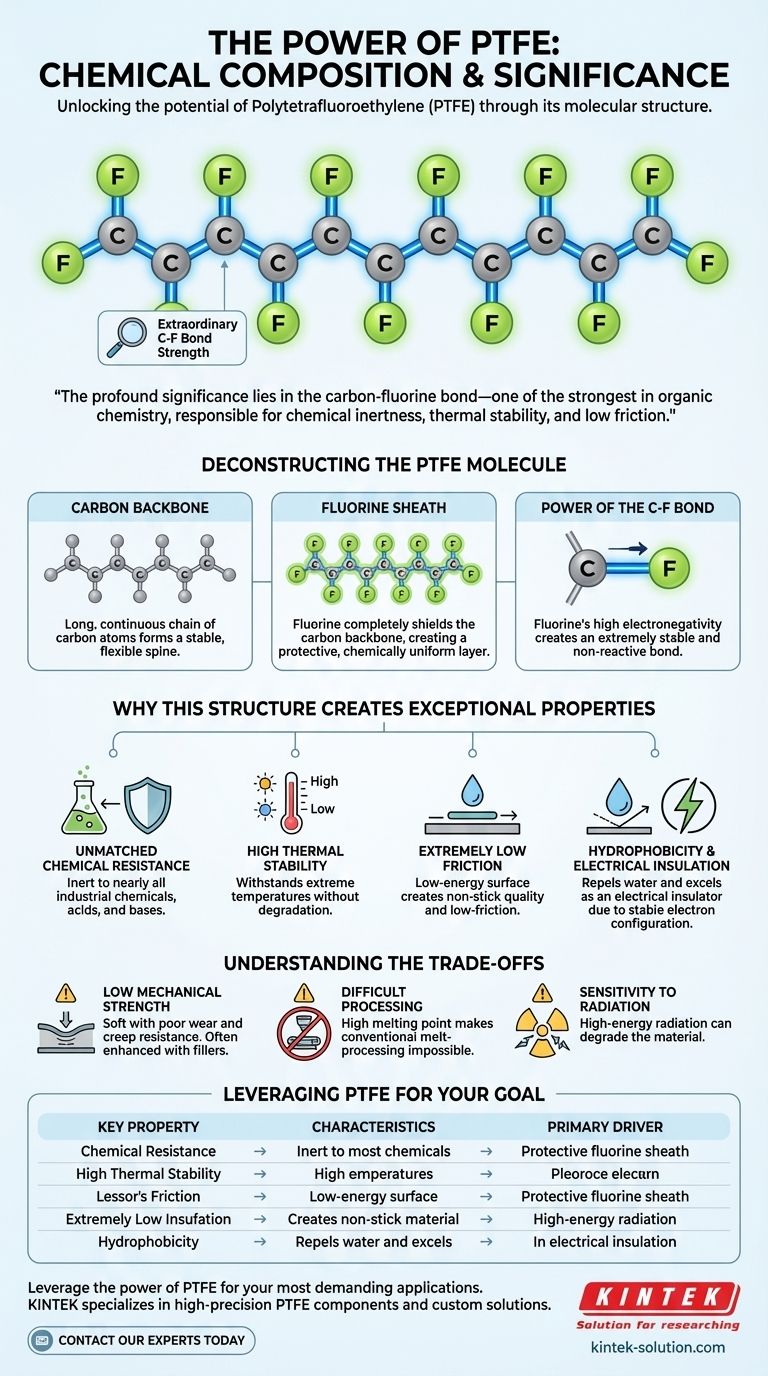
At its core, Polytetrafluoroethylene (PTFE) is a remarkably simple synthetic polymer. It is a fluorocarbon, composed exclusively of carbon and fluorine atoms. Its structure consists of a long, linear chain of carbon atoms, with each carbon atom being bonded to two fluorine atoms, creating a stable and uniform molecule.
The profound significance of PTFE's composition lies in the exceptional strength of the carbon-fluorine (C-F) bond. This bond is one of the strongest in organic chemistry, and it is directly responsible for the material's signature properties: extreme chemical inertness, high thermal stability, and very low friction.

Deconstructing the PTFE Molecule
To understand why PTFE behaves the way it does, we must examine its molecular architecture. The material's power is not derived from complexity, but from the robust simplicity of its repeating units.
The Carbon Backbone
The foundation of the PTFE molecule is a long, continuous chain of carbon atoms linked together. This forms a stable, flexible spine for the polymer.
The Fluorine Sheath
The critical feature is that this carbon backbone is completely encased by fluorine atoms. Each carbon atom in the chain is shielded by two fluorine atoms, creating a tight, protective, and chemically uniform outer layer.
The Power of the Carbon-Fluorine Bond
The bond between each carbon and fluorine atom is extraordinarily strong and stable. Fluorine is the most electronegative element, meaning it holds onto its electrons very tightly, which contributes to the strength and non-reactivity of the bond. This molecular stability is the source of all of PTFE's most valued characteristics.
Why This Structure Creates Exceptional Properties
The unique arrangement of carbon and fluorine imparts a combination of properties that are difficult to achieve in other materials. It is a direct consequence of its chemical makeup.
Unmatched Chemical Resistance
The protective fluorine sheath effectively isolates the carbon backbone from chemical attack. This makes PTFE inert to nearly all industrial chemicals, acids, and bases, making it an essential material for seals, gaskets, and linings in corrosive environments.
High Thermal Stability
Breaking the strong carbon-fluorine bonds requires a significant amount of thermal energy. As a result, PTFE can withstand a very wide range of temperatures without degrading, maintaining its properties in both cryogenic and high-heat applications.
Extremely Low Friction
The surface of PTFE is composed entirely of fluorine atoms. This creates a surface with very low energy, meaning other substances have little to nothing to adhere to. This is the principle behind its famous "non-stick" quality and its use in low-friction bearings.
Hydrophobicity and Electrical Insulation
The same low-energy surface that repels other materials also repels water, making PTFE highly hydrophobic. Furthermore, the stable electron configuration of the C-F bonds makes it an excellent electrical insulator, as there are no free electrons to conduct a current.
Understanding the Trade-offs
While its chemical composition provides incredible advantages, it also introduces certain limitations that are crucial for any technical professional to understand.
Relatively Low Mechanical Strength
Compared to many engineering plastics, PTFE is soft and has poor resistance to wear and creep (deformation under load). Its mechanical properties are often enhanced by adding fillers like glass, carbon, or bronze.
Difficult Processing
PTFE's high melting point and extremely high melt viscosity make it impossible to process using conventional melt-processing techniques like injection molding. It requires specialized methods such as compression molding and sintering.
Sensitivity to Radiation
While resistant to chemicals and heat, PTFE can be degraded by high-energy radiation, such as gamma rays. This radiation can break the carbon-fluorine bonds, compromising the material's integrity.
Leveraging PTFE's Composition for Your Goal
Understanding the link between PTFE's composition and its properties allows you to specify it with confidence.
- If your primary focus is chemical inertness: PTFE's fluorine-shielded backbone makes it the default choice for seals, linings, and components exposed to corrosive substances.
- If your primary focus is low friction: Its low-energy surface is ideal for creating non-stick coatings or self-lubricating bearings where smooth motion is critical.
- If your primary focus is performance at extreme temperatures: PTFE's stable C-F bonds ensure reliability in applications from cryogenic systems to high-temperature electrical insulation.
Ultimately, the power of PTFE comes from how its simple, two-element composition creates a molecule with an unparalleled defense against chemical and thermal attack.
Summary Table:
| Property | Key Characteristic | Primary Driver |
|---|---|---|
| Chemical Resistance | Inert to most chemicals | Protective fluorine sheath |
| Thermal Stability | Stable from cryogenic to high temps | Strong carbon-fluorine bonds |
| Low Friction | Excellent non-stick properties | Low-energy fluorine surface |
| Electrical Insulation | Excellent dielectric properties | Stable electron configuration |
Leverage the power of PTFE for your most demanding applications.
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise in custom fabrication, from prototypes to high-volume orders, ensures you get a component that perfectly balances PTFE's inherent properties with the mechanical strength required for your specific use case.
Contact our experts today to discuss how our precision PTFE solutions can solve your unique challenges.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the key benefits of PTFE in custom fabrication? Unlock Performance in Extreme Conditions
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications
- Why is PTFE suitable for cryogenic or high-temperature applications? Unmatched Thermal Stability from -450°F to 500°F
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the base characteristics of PTFE? Unlocking Extreme Performance in Friction, Temperature, and Chemical Resistance



















