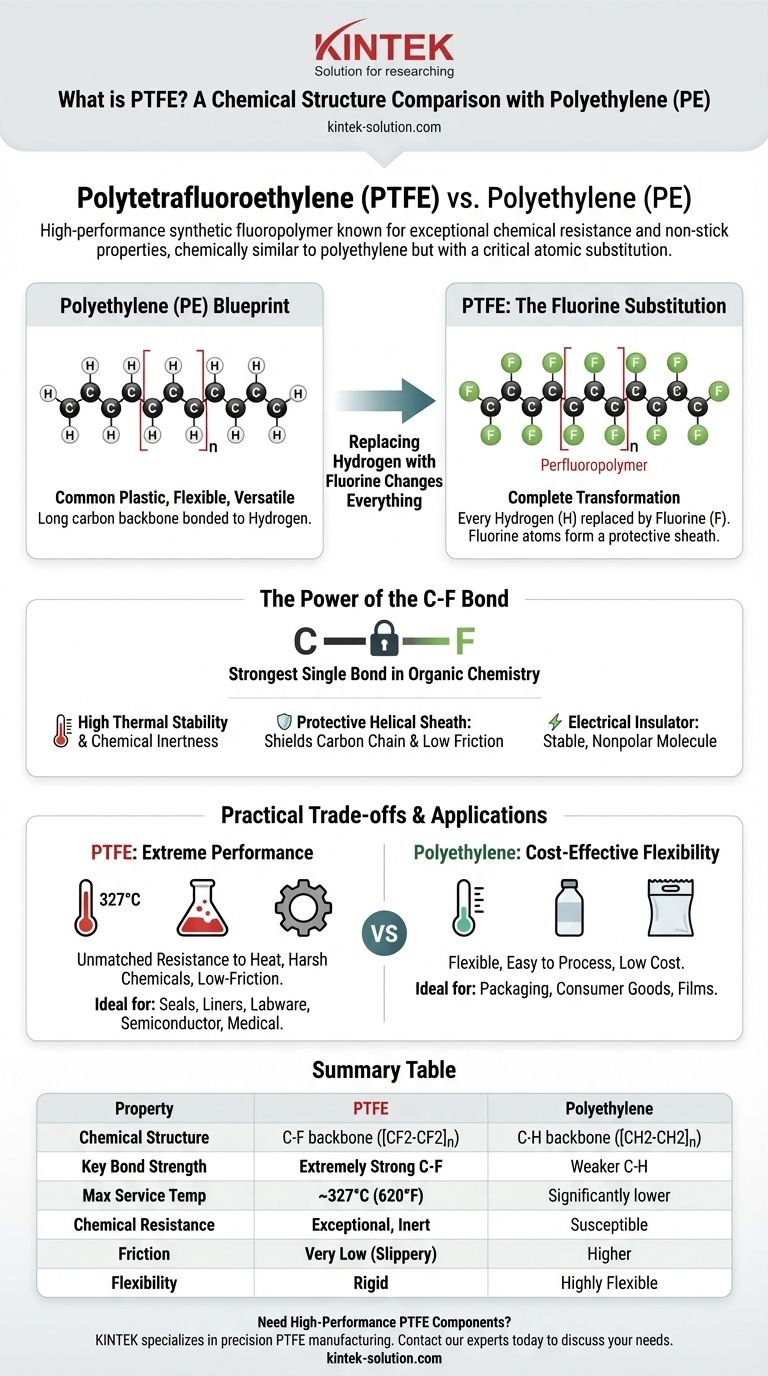
Polytetrafluoroethylene (PTFE) is a high-performance synthetic fluoropolymer known for its exceptional chemical resistance and non-stick properties. Its chemical structure is remarkably similar to the common plastic polyethylene (PE), consisting of a long carbon backbone. The critical difference is that in PTFE, every hydrogen atom found in polyethylene has been replaced by a fluorine atom.
The simple substitution of small hydrogen atoms with larger, highly electronegative fluorine atoms creates an incredibly strong carbon-fluorine bond and a protective molecular "sheath." This single structural change is the direct cause of PTFE's famous properties, transforming a common plastic blueprint into a material capable of withstanding extreme environments.

From Polyethylene to PTFE: A Structural Comparison
To understand what makes PTFE unique, we must first look at its simpler cousin, polyethylene. This comparison reveals how a subtle change in atomic makeup can lead to a radical difference in material performance.
The Polyethylene Blueprint
Polyethylene is one of the most common plastics in the world. Its structure is a long chain of carbon atoms, with each carbon atom bonded to two hydrogen atoms. This forms the repeating unit [CH2-CH2]n. It is a relatively simple, flexible, and versatile material.
The Fluorine Substitution
PTFE starts with the same carbon backbone as polyethylene but undergoes a complete transformation. Every hydrogen (H) atom is replaced by a fluorine (F) atom, creating the repeating unit [CF2-CF2]n. Because it is fully saturated with fluorine, it is known as a perfluoropolymer.
Why Replacing Hydrogen with Fluorine Changes Everything
The switch from hydrogen to fluorine is not a minor detail; it is the fundamental reason for PTFE's elite performance characteristics. This substitution affects the polymer at a molecular level in three critical ways.
The Strength of the Carbon-Fluorine Bond
The bond between a carbon atom and a fluorine atom (C-F) is one of the strongest single bonds in organic chemistry. This immense bond strength requires a great deal of energy to break, which directly translates into PTFE's high thermal stability and chemical inertness.
The Protective Fluorine "Sheath"
Fluorine atoms are significantly larger than hydrogen atoms. In the PTFE molecule, these larger atoms pack tightly around the carbon backbone, forming a protective helical sheath. This sheath physically shields the carbon chain from attack by corrosive chemicals.
Furthermore, this uniform, negatively charged sheath creates very weak forces between adjacent molecules. This intermolecular repulsion is the source of PTFE's exceptionally low coefficient of friction, making it one of the most slippery substances known.
The Impact on Electrical Properties
Fluorine atoms have a very strong attraction to electrons. This high electronegativity results in a stable, nonpolar molecule. This lack of electrical polarity makes PTFE an outstanding electrical insulator, preventing the flow of current.
Understanding the Practical Trade-offs
While structurally similar, the resulting properties of PTFE and PE make them suitable for completely different applications. Understanding their trade-offs is crucial for material selection.
Thermal and Chemical Stability
PTFE is the undisputed winner in harsh environments. It is resistant to nearly all chemicals and solvents and has a very high melting point of around 327°C (620°F). Polyethylene, by contrast, has a much lower melting point and is susceptible to many common chemicals.
Mechanical Properties and Flexibility
While PTFE has superior mechanical strength and resistance to creep (slow deformation under stress), its primary trade-off is rigidity. Polyethylene is far more flexible and easier to process, making it ideal for applications like films, bags, and flexible containers.
Making the Right Choice for Your Application
The decision between PTFE and polyethylene hinges entirely on the demands of the operating environment.
- If your primary focus is extreme performance: PTFE is the necessary choice for its unmatched resistance to high temperatures, harsh chemicals, and for applications requiring a low-friction surface.
- If your primary focus is cost-effective flexibility: Polyethylene is the superior option for its ease of processing, low cost, and flexibility in everyday applications like packaging and consumer goods.
Understanding that this profound performance difference stems from a simple atomic substitution is the key to leveraging the distinct capabilities of each material.
Summary Table:
| Property | PTFE (Polytetrafluoroethylene) | Polyethylene (PE) |
|---|---|---|
| Chemical Structure | Carbon backbone with Fluorine atoms ([CF2-CF2]n) | Carbon backbone with Hydrogen atoms ([CH2-CH2]n) |
| Key Bond Strength | Extremely strong Carbon-Fluorine (C-F) bond | Weaker Carbon-Hydrogen (C-H) bond |
| Max Service Temperature | ~327°C (620°F) | Significantly lower |
| Chemical Resistance | Exceptional; inert to nearly all chemicals | Susceptible to many chemicals |
| Coefficient of Friction | Very low (slippery) | Higher |
| Flexibility | Rigid | Highly flexible |
Need High-Performance PTFE Components for Demanding Applications?
At KINTEK, we specialize in the precision manufacturing of PTFE components—including seals, liners, and custom labware. Our expertise ensures you get the full benefit of PTFE's superior chemical resistance, thermal stability, and non-stick properties for your projects in the semiconductor, medical, laboratory, and industrial sectors.
We offer custom fabrication from prototypes to high-volume orders, tailoring solutions to your exact specifications. Let us help you leverage the right material for extreme performance.
Contact our experts today to discuss your PTFE needs!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- PTFE Chemical Solvent Sampling Spoon
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
People Also Ask
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs



















