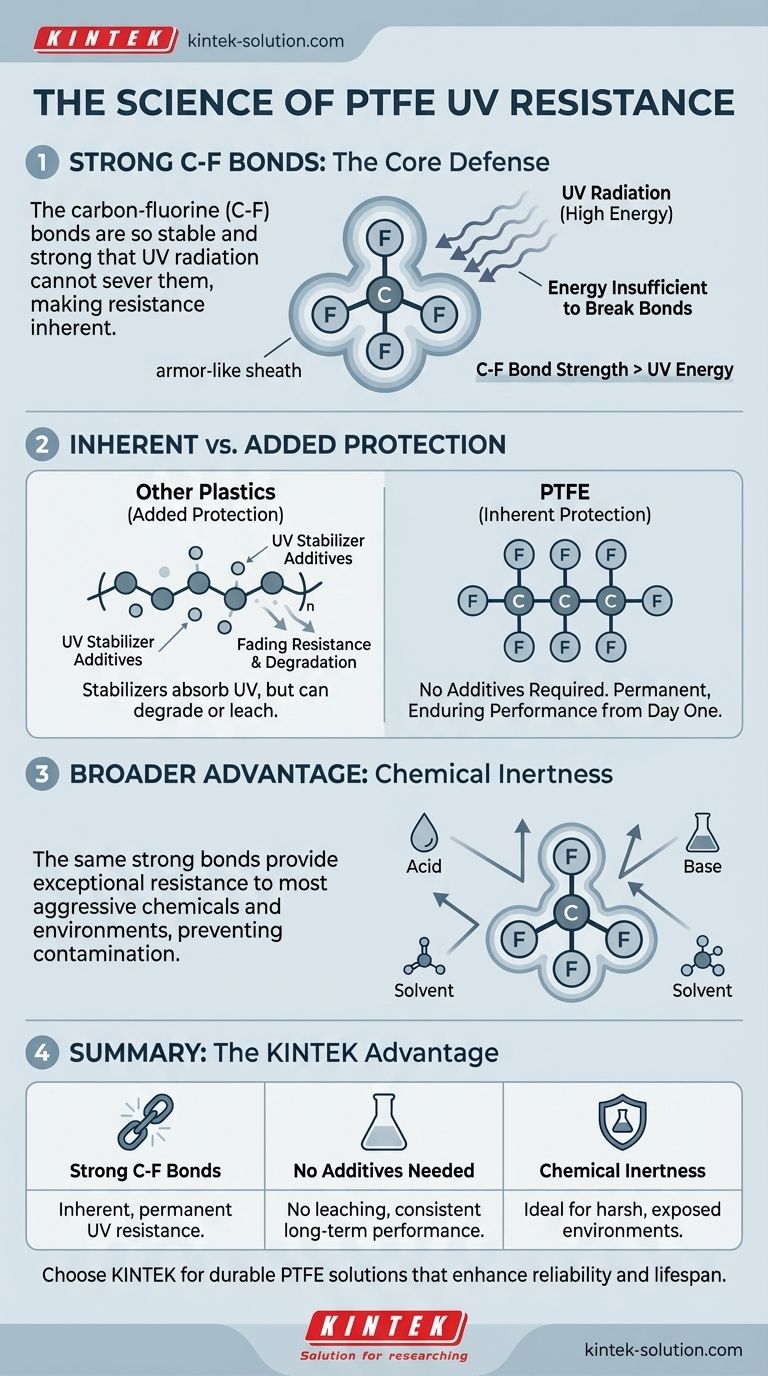
The reason for PTFE's exceptional UV resistance lies in the immense strength of its carbon-fluorine (C-F) bonds. Ultraviolet radiation is a form of high-energy light that degrades many materials by breaking their molecular bonds. However, the C-F bonds that form the backbone of Polytetrafluoroethylene (PTFE) are so stable and strong that the energy from UV radiation is insufficient to break them.
The core principle is simple: The chemical bonds holding PTFE together are stronger than the energy UV radiation can deliver. This makes the material inherently immune to UV degradation, a property that is built into its very molecular structure, not added later.

The Science of Stability: Bonds vs. Energy
To understand why PTFE endures where other materials fail, we must look at the interaction between energy and molecular structure. It's a fundamental battle, and PTFE is uniquely equipped to win.
Understanding UV Degradation
Ultraviolet radiation from the sun is a primary cause of material breakdown. This high-energy light acts like a microscopic hammer, striking the chemical bonds that hold a polymer together.
When these bonds break, the material's properties change. It can lead to discoloration, brittleness, loss of strength, and eventual failure. Many common plastics are susceptible to this form of attack.
The Strength of the Carbon-Fluorine Bond
The defining characteristic of PTFE is the carbon-fluorine (C-F) bond. This bond is one of the strongest known single bonds in organic chemistry.
The fluorine atoms effectively create an armor-like sheath around the carbon backbone of the polymer chain. This sheath is exceptionally stable and difficult to disrupt.
Why UV Radiation Fails
The energy contained within UV light simply isn't powerful enough to sever the robust C-F bonds in PTFE.
Because the molecular chain remains intact and undamaged by the radiation, the material does not degrade. Its physical and chemical properties remain unchanged even after prolonged exposure.
A Broader Consequence: Extreme Chemical Inertness
The same molecular stability that provides UV resistance also makes PTFE one of the most chemically resistant materials known. This is not a coincidence; it is a direct result of the same underlying principle.
The Same Principle of Defense
Just as UV energy cannot break the C-F bonds, most aggressive chemicals also lack the ability to react with and break them down.
Acids, bases, solvents, and even highly corrosive substances are unable to find a weak point to initiate a chemical attack. This makes PTFE stable in nearly any chemical environment.
The Few Exceptions
Only a handful of the most aggressive substances can affect PTFE, typically under specific conditions of high temperature and pressure. These include molten alkali metals and potent fluorinating agents like chlorine trifluoride.
The Practical Advantage: No Additives Required
Many polymers require chemical additives, known as UV stabilizers, to survive outdoors. PTFE does not, which provides a significant long-term advantage.
Inherent vs. Added Protection
In other plastics, UV resistance is a borrowed property. Stabilizers are mixed in to absorb or dissipate UV energy, protecting the weaker polymer bonds.
PTFE's protection is inherent. It doesn't need a separate chemical to do the job because its own structure provides complete defense.
The Risk of Leaching
Additives in other materials can leach out or degrade over time, causing the material's UV resistance to fade. This leads to unpredictable performance over the product's lifespan.
PTFE's Enduring Performance
Because its UV resistance is a fundamental part of its molecular structure, it does not diminish. The performance you get on day one is the same performance you will have years later, making it an exceptionally reliable choice for long-term outdoor applications.
Making the Right Choice for Your Application
Understanding the source of PTFE's stability allows you to apply it correctly.
- If your primary focus is maximum UV and chemical endurance: PTFE is an unparalleled choice because its stability is an inseparable part of its molecular makeup.
- If your primary concern is long-term reliability in exposed environments: PTFE's additive-free nature ensures its UV resistance will not degrade, offering predictable, life-long performance.
- If you need a material that will not react with its environment: The same C-F bond that resists UV also makes PTFE inert, preventing contamination and ensuring purity in sensitive applications.
Ultimately, choosing PTFE is a decision to rely on fundamental chemical stability rather than temporary additives.
Summary Table:
| Key Factor | Explanation | Benefit |
|---|---|---|
| Strong C-F Bonds | The carbon-fluorine bonds are stronger than the energy of UV radiation. | Inherent, permanent UV resistance. |
| No Additives Needed | Stability is built into the molecular structure, not added as a coating. | No risk of leaching or performance degradation over time. |
| Chemical Inertness | The same strong bonds provide exceptional resistance to chemicals. | Ideal for harsh, exposed environments and sensitive applications. |
Need components that won't degrade under UV light or harsh chemicals?
KINTEK specializes in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise in custom fabrication, from prototypes to high-volume orders, ensures you get parts with inherent, long-lasting stability.
Contact KINTEK today to discuss how our durable PTFE solutions can enhance your project's reliability and lifespan.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss



















