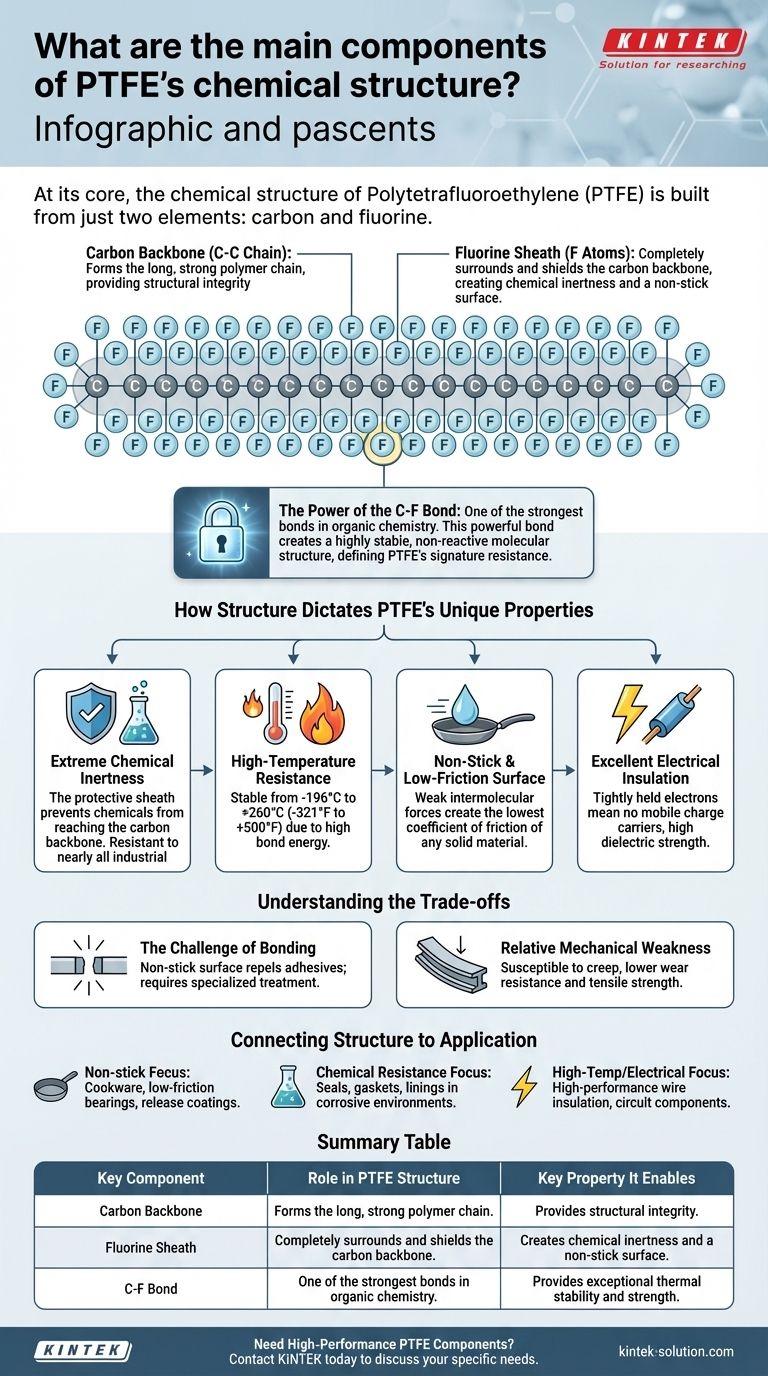
At its core, the chemical structure of Polytetrafluoroethylene (PTFE) is built from just two elements: carbon and fluorine. This simple composition forms a polymer chain where a long backbone of carbon atoms is completely surrounded by a dense sheath of fluorine atoms. It is this unique and stable arrangement that gives PTFE its remarkable collection of properties.
The exceptional strength of the Carbon-Fluorine (C-F) bond is the single most important factor defining PTFE. This powerful bond creates a highly stable, non-reactive molecular structure that is directly responsible for PTFE's signature resistance to heat, chemicals, and surface adhesion.

Deconstructing the PTFE Molecule
To understand why PTFE behaves the way it does, we must look at how its two components are arranged. The structure is elegant in its simplicity and effectiveness.
The Carbon Backbone
Like many common polymers, PTFE starts with a long, repeating chain of carbon atoms (C) bonded to one another. This chain provides the fundamental structure and length of the polymer molecule.
The Fluorine Sheath
The critical feature of PTFE is that each carbon atom in the backbone is also bonded to two fluorine (F) atoms. Because fluorine atoms are relatively large and highly electronegative, they wrap tightly around the carbon backbone.
This arrangement creates a dense, uniform, and electrically neutral outer surface, effectively shielding the vulnerable carbon backbone from any outside interaction.
The Power of the Carbon-Fluorine Bond
The bond between a carbon atom and a fluorine atom is one of the strongest known single bonds in organic chemistry. It requires a tremendous amount of energy to break, which is the primary source of PTFE's extreme stability.
How Structure Dictates PTFE's Unique Properties
Every famous characteristic of PTFE can be traced directly back to the strength and arrangement of its Carbon-Fluorine bonds.
Extreme Chemical Inertness
The protective fluorine sheath prevents chemicals and solvents from reaching the carbon backbone. Combined with the immense strength of the C-F bonds, this makes the molecule almost completely inert and resistant to nearly all industrial chemicals, acids, and alkalis.
High-Temperature Resistance
The energy required to break the C-F bonds is exceptionally high. This translates directly into outstanding thermal stability, allowing PTFE to perform consistently across a vast temperature range, typically from -196°C to +260°C (-321°F to +500°F).
Non-Stick and Low-Friction Surface
The fluorine atoms on the surface of the molecule create very weak intermolecular forces. There is almost nothing for other materials to "grab onto," which is why substances slide right off. This gives PTFE the lowest coefficient of friction of any known solid material—even lower than wet ice on wet ice.
Excellent Electrical Insulation
The electrons within the C-F bonds are held very tightly by the fluorine atoms. This leaves no mobile electrons to carry an electrical current, making PTFE an exceptional electrical insulator with high dielectric strength.
Understanding the Trade-offs
The same properties that make PTFE so valuable also introduce specific limitations that are crucial to understand.
The Challenge of Bonding
The low-friction, non-stick surface that repels everything also repels adhesives. Bonding PTFE to other materials is notoriously difficult and often requires specialized surface treatments like chemical etching to create a bondable surface.
Relative Mechanical Weakness
While chemically and thermally robust, PTFE is a relatively soft material. It can be susceptible to "creep" (slow deformation under sustained load) and has lower wear resistance and tensile strength compared to other engineering plastics.
Connecting Structure to Application
Understanding the molecular foundation helps you determine precisely where PTFE will excel.
- If your primary focus is non-stick surfaces or low friction: The uniform fluorine sheath is the direct cause, making PTFE ideal for cookware, low-friction bearings, and release coatings.
- If your primary focus is chemical resistance: The powerful C-F bond and protective sheath make it the top choice for seals, gaskets, and linings in corrosive environments.
- If your primary focus is high-temperature or electrical insulation: The sheer stability of the C-F bond makes it perfect for high-performance wire insulation, circuit components, and other demanding electronic applications.
By recognizing that PTFE's power comes from its simple two-element structure, you can confidently deploy it to solve your most challenging material science problems.
Summary Table:
| Key Component | Role in PTFE Structure | Key Property It Enables |
|---|---|---|
| Carbon Backbone | Forms the long, strong polymer chain | Provides structural integrity |
| Fluorine Sheath | Completely surrounds and shields the carbon backbone | Creates chemical inertness and a non-stick surface |
| C-F Bond | One of the strongest bonds in organic chemistry | Provides exceptional thermal stability and strength |
Need High-Performance PTFE Components?
Understanding the molecular structure of PTFE is the first step. Applying that knowledge to solve real-world challenges is the next. KINTEK specializes in manufacturing precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors.
Whether you require a standard part or a custom-fabricated solution from prototype to high-volume production, our expertise ensures you get the chemical resistance, thermal stability, and non-stick performance your application demands.
Contact KINTERO today to discuss your specific PTFE needs and leverage our material science expertise for your project.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments
- What industrial applications does PTFE have? Unlock Performance in Extreme Environments



















