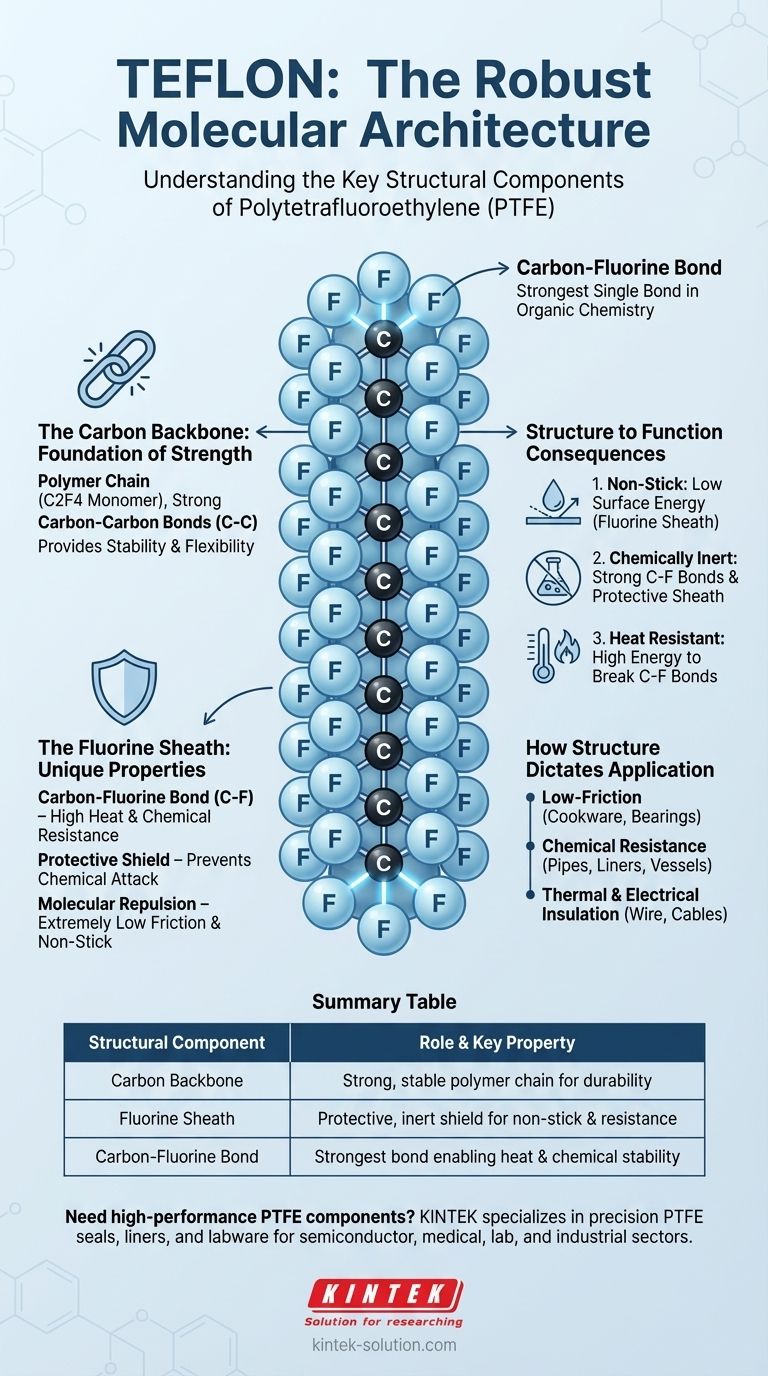
At its core, Teflon is a simple yet remarkably robust polymer. Its technical name is Polytetrafluoroethylene (PTFE), and its structure consists of a long, repeating chain of carbon atoms completely surrounded by fluorine atoms. This foundational structure is built from its basic monomer unit, tetrafluoroethylene (C2F4).
The key to understanding Teflon is to see its structure as a stable carbon backbone protected by a chemically inert and non-stick "sheath" of fluorine atoms. The exceptionally strong bond between carbon and fluorine is what gives Teflon its famous properties.

The Carbon Backbone: A Foundation of Strength
The entire structure of Teflon is built upon a long, continuous chain of carbon atoms. This chain provides the underlying stability and strength for the material.
The Polymer Chain
Teflon is a polymer, which means it is a large molecule made of many smaller, identical units linked together. In this case, the repeating unit is C2F4, forming a chain that can be thousands of atoms long.
The Carbon-Carbon Bond
The carbon atoms are linked to each other with strong covalent bonds. This creates a stable and durable "spine" for the polymer, contributing to its overall toughness and flexibility mentioned in technical specifications.
The Fluorine Sheath: The Source of Its Unique Properties
While the carbon chain provides the backbone, it's the fluorine atoms that give Teflon its extraordinary characteristics. These atoms completely encase the carbon chain.
The Carbon-Fluorine Bond
The bond between each carbon atom and each fluorine atom is one of the strongest single bonds in organic chemistry. It requires a massive amount of energy to break, which is the primary reason Teflon is so resistant to heat and chemical attack.
A Shield of Fluorine Atoms
The fluorine atoms are larger than the carbon atoms they are attached to. Because of this, they pack tightly together, forming a protective, seamless shield around the entire carbon backbone. This "fluorine sheath" physically prevents other chemicals from reaching and reacting with the vulnerable carbon chain.
Molecular Repulsion
Fluorine is the most electronegative element, meaning it strongly pulls electrons toward itself. This creates a uniform negative charge on the surface of the Teflon molecule, which repels almost all other molecules. This electrical repulsion is the fundamental reason for Teflon's extremely low friction and non-stick surface.
Understanding the Consequences: From Structure to Function
The specific arrangement of carbon and fluorine atoms directly translates into the valuable properties Teflon is known for. Understanding the structure makes it clear why it behaves the way it does.
Why It's Non-Stick
The tightly packed, negatively charged fluorine sheath creates a surface with extremely low energy. Other substances, like water or oil, have little to no molecular attraction to this surface, causing them to bead up and slide off easily.
Why It's Chemically Inert
For a chemical reaction to occur, a substance must be able to break the bonds within the Teflon molecule. The combination of the immensely strong C-F bonds and the protective fluorine sheath makes it nearly impossible for acids, bases, or solvents to attack the polymer chain.
Why It Resists High Temperatures
The strength of the carbon-fluorine bond means that a great deal of thermal energy is required to break the molecule apart. This is why Teflon maintains its structural integrity at high temperatures where other polymers would melt or decompose.
How Structure Dictates Application
The direct link between Teflon's molecular structure and its physical properties determines its ideal use cases.
- If your primary focus is a low-friction surface: The non-stick, repulsive quality of the fluorine sheath makes it ideal for cookware, bearings, and other applications where minimizing friction is critical.
- If your primary focus is chemical resistance: The inert nature of the fluorine sheath and the strength of the C-F bond make it the gold standard for lining pipes, vessels, and containers that handle highly corrosive chemicals.
- If your primary focus is thermal and electrical insulation: Its ability to withstand high temperatures and its non-conductive properties make it a superior material for insulating high-performance wires and cables.
Ultimately, Teflon's simple, repeating structure of carbon and fluorine is a masterclass in how molecular architecture can create materials with truly exceptional capabilities.
Summary Table:
| Structural Component | Role & Key Property |
|---|---|
| Carbon Backbone | Provides a strong, stable polymer chain for durability and flexibility. |
| Fluorine Sheath | Creates a protective, inert shield responsible for non-stick and chemical resistance. |
| Carbon-Fluorine Bond | One of the strongest bonds in chemistry, enabling exceptional heat and chemical stability. |
Need high-performance PTFE components that leverage this robust molecular structure?
At KINTEK, we specialize in manufacturing precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise in custom fabrication, from prototypes to high-volume orders, ensures you get parts that deliver on Teflon's full potential of chemical inertness, low friction, and thermal stability.
Contact our experts today to discuss your specific application requirements.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
People Also Ask
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- What are the key advantages of PTFE? Unmatched Performance for Extreme Environments
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability



















