At its core, Polytetrafluoroethylene (PTFE) is one of the most chemically inert polymers available. Its resistance stems from the incredibly strong carbon-fluorine bonds that form its molecular backbone. This structure makes PTFE virtually impervious to a vast spectrum of chemicals, including aggressive acids, bases, solvents, and oxidizing agents, even at high temperatures.
While PTFE offers near-universal chemical resistance for most industrial and laboratory applications, its integrity is compromised by a very specific and limited class of substances: molten alkali metals and highly reactive fluorine compounds.

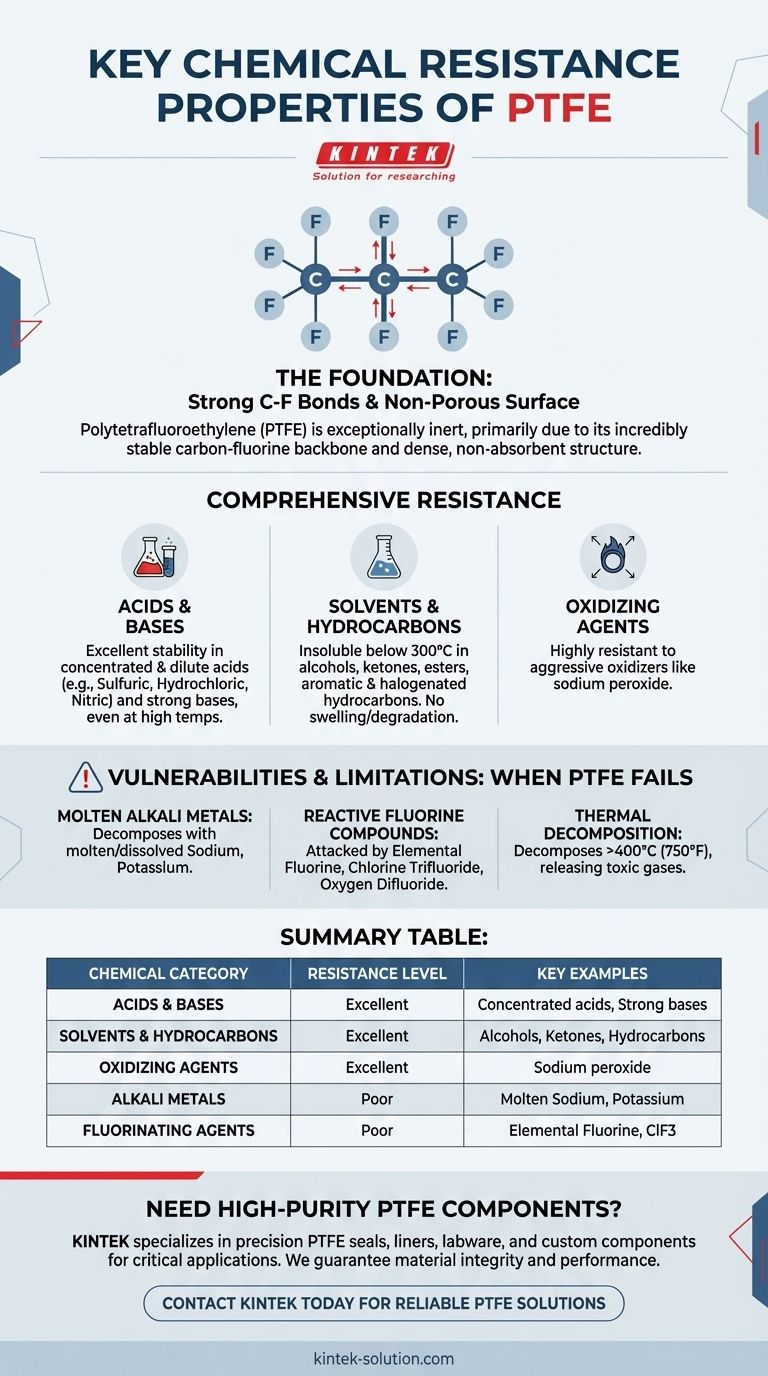
The Foundation of PTFE's Chemical Inertness
To understand PTFE's capabilities, we must first look at its unique molecular structure. Its properties are not incidental; they are a direct result of its chemical composition.
The Strength of the Carbon-Fluorine Bond
PTFE is composed of long chains of carbon atoms, where each carbon is completely sheathed by fluorine atoms.
The bond between carbon and fluorine is exceptionally strong and stable. This powerful bond protects the vulnerable carbon backbone from chemical attack, rendering the material non-reactive.
A Non-Porous, Non-Absorbent Surface
PTFE's surface is dense and non-porous. This physical characteristic prevents chemicals from being absorbed into the material.
This non-absorbent nature is critical in high-purity applications, as it minimizes the risk of leaching, material degradation, or cross-contamination between processes.
A Comprehensive Breakdown of Resistance
PTFE remains unchanged when exposed to the vast majority of common and aggressive chemical agents.
Superior Resistance to Acids and Bases
PTFE exhibits excellent stability in both dilute and concentrated acids, including sulfuric, hydrochloric, and nitric acid, even when boiling. It is equally resistant to strong bases.
Inertness to Solvents and Hydrocarbons
PTFE is famously insoluble in all known solvents below 300°C.
It can be used confidently with alcohols, aldehydes, ketones, esters, and various aliphatic, aromatic, and halogenated hydrocarbons without swelling, dissolving, or degrading.
Stability Against Oxidizing Agents
The material is highly resistant to potent oxidizing agents, making it suitable for applications involving materials like sodium peroxide and other aggressive oxidizers.
Understanding the Trade-offs: When PTFE Fails
No material is perfect. While PTFE's list of resistances is long, its specific vulnerabilities are critical to understand for safe and effective material selection.
The Threat of Molten Alkali Metals
The primary exception to PTFE's chemical resistance is molten or dissolved alkali metals, such as sodium and potassium. These highly reactive metals can attack the polymer and cause it to decompose.
High-Reactivity Fluorine Compounds
Certain fluorine-based compounds can also attack PTFE, especially at high temperatures and pressures. These include elementary fluorine gas, chlorine trifluoride, and oxygen difluoride.
Thermal Decomposition Limits
PTFE is stable over a wide temperature range but will begin to decompose at temperatures around 400°C (750°F). This decomposition can release toxic fluorocarbon gases, a significant safety consideration in high-temperature designs.
Making the Right Choice for Your Application
Selecting the correct material requires matching its properties to the operational environment.
- If your primary focus is handling aggressive acids, bases, or organic solvents: PTFE is the default choice for ensuring system integrity and longevity.
- If your primary focus is high-purity or sanitary processes: PTFE's non-leaching, non-absorbent, and physiologically inert properties prevent contamination.
- If your environment involves molten alkali metals or potent fluorinating agents: You must avoid PTFE and specify alternative materials designed for these unique conditions.
By understanding both its remarkable strengths and its specific limitations, you can leverage PTFE to build exceptionally reliable and safe systems.
Summary Table:
| Chemical Category | PTFE Resistance Level | Key Examples |
|---|---|---|
| Acids & Bases | Excellent | Concentrated sulfuric, hydrochloric, nitric acids; strong bases |
| Solvents & Hydrocarbons | Excellent | Alcohols, ketones, esters, aromatic hydrocarbons |
| Oxidizing Agents | Excellent | Sodium peroxide, other aggressive oxidizers |
| Alkali Metals | Poor | Molten or dissolved sodium, potassium |
| Fluorinating Agents | Poor | Elemental fluorine, chlorine trifluoride |
Need high-purity, chemically inert PTFE components for your critical application?
KINTEK specializes in manufacturing precision PTFE seals, liners, labware, and custom components for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your systems benefit from PTFE's superior chemical resistance while avoiding its limitations.
We offer custom fabrication from prototypes to high-volume orders, guaranteeing the material integrity and performance your processes demand.
Contact KINTEK today to discuss your specific requirements and receive a quote for reliable PTFE solutions.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
People Also Ask
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE



















