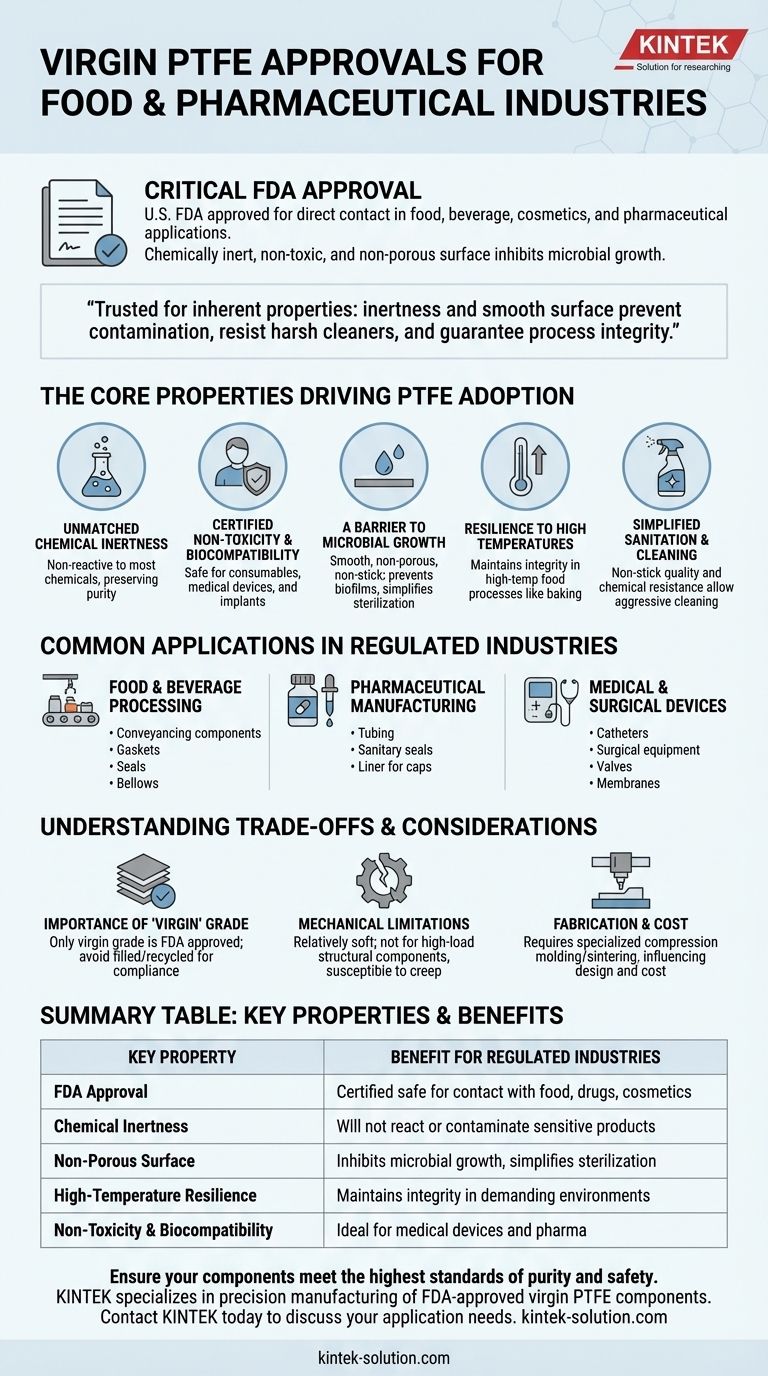
Virgin Polytetrafluoroethylene (PTFE) holds a critical approval from the U.S. Food and Drug Administration (FDA) for use in the food, beverage, cosmetics, and pharmaceutical industries. This clearance is granted because virgin PTFE is chemically inert, non-toxic, and features a non-porous surface that inhibits microbial growth, making it a benchmark material for applications demanding the highest levels of purity and safety.
The core reason virgin PTFE is trusted in food and pharmaceutical settings is not just its FDA approval, but the inherent properties that earn it. Its chemical inertness and smooth surface ensure it will not contaminate products, react with harsh cleaning agents, or harbor bacteria, thus guaranteeing process integrity.

The Core Properties Driving PTFE Adoption
The suitability of virgin PTFE for sensitive industries is not based on a single attribute but on a combination of powerful characteristics. These properties work together to meet the stringent demands of sanitary environments.
Unmatched Chemical Inertness
Virgin PTFE is one of the most non-reactive materials known. It does not react with the vast majority of industrial chemicals, food products, or potent pharmaceutical compounds.
This inertness is crucial because it ensures the material will not degrade or leach substances into the product, preserving its intended purity and safety.
Certified Non-Toxicity and Biocompatibility
The FDA's approval confirms that virgin PTFE is non-toxic and safe for direct contact with consumables and medical products.
Its biocompatibility also makes it suitable for use in medical devices and surgical equipment, where materials must not cause adverse reactions within the body.
A Barrier to Microbial Growth
The surface of virgin PTFE is exceptionally smooth and non-porous. It lacks the microscopic pits and crevices where bacteria and other microbes can attach and colonize.
This physical characteristic, combined with its non-stick nature, makes it difficult for biofilms to form and simplifies cleaning and sterilization.
Resilience to High Temperatures
PTFE maintains its integrity over a wide temperature range. This allows it to be used in high-temperature processes common in food production, such as baking and drying ovens, without degrading.
Simplified Sanitation and Cleaning
The famous non-stick quality of PTFE prevents product residue from adhering to surfaces. This property, along with its chemical resistance, allows for aggressive cleaning and sterilization protocols using harsh chemicals without damaging the material.
Common Applications in Regulated Industries
Thanks to its unique properties, FDA-approved virgin PTFE is fabricated into a wide array of components used across food, pharmaceutical, and medical fields.
Food and Beverage Processing
In this sector, PTFE is commonly found in conveyancing components like guide rails, profiles, and slides that require low friction and easy cleanup. It is also used for gaskets, seals, and bellows in processing machinery to ensure a pure and leak-free environment.
Pharmaceutical Manufacturing
The pharmaceutical industry relies on PTFE for tubing to transfer corrosive chemicals, sanitary seals and gaskets in reaction vessels, and liners for caps to prevent any interaction between the container and its sensitive contents.
Medical and Surgical Devices
Due to its biocompatibility and inertness, PTFE is a key material in medical applications. It is used in catheters, surgical equipment, valves, and even as membranes in specific treatments like glaucoma surgery.
Understanding the Trade-offs and Considerations
While virgin PTFE is an exceptional material, its proper application requires an understanding of its specific characteristics and limitations.
The Importance of "Virgin" Grade
The FDA approval and associated safety benefits apply specifically to virgin PTFE. Filled or recycled grades of PTFE may contain additives that are not approved for food or pharmaceutical contact. Verifying the material grade is essential for compliance.
Mechanical Limitations
PTFE is a relatively soft material with lower resistance to wear and "creep" (deformation under sustained load) compared to other engineering plastics. It is not an ideal choice for high-load structural components where mechanical strength is the primary requirement.
Fabrication and Cost
PTFE cannot be processed using conventional melt-processing techniques like injection molding. It typically requires specialized compression molding and sintering or machining from stock shapes, which can influence component design and overall cost.
Making the Right Choice for Your Goal
Selecting the right material is a critical decision in any regulated industry. Virgin PTFE offers a powerful solution when its properties align with your primary needs.
- If your primary focus is product purity and non-contamination: Virgin PTFE is an ideal choice for any surface that directly contacts food, drugs, or sensitive chemicals.
- If your application involves aggressive cleaning cycles or sterilization: PTFE's resistance to harsh chemicals and its easily sanitized surface make it a highly reliable and durable material.
- If your component requires high structural strength or abrasion resistance: Carefully evaluate whether virgin PTFE's mechanical properties are sufficient for the load, or consider alternative materials for demanding structural parts.
Ultimately, relying on FDA-approved virgin PTFE provides a proven foundation for safety, compliance, and operational integrity in the most demanding sanitary environments.
Summary Table:
| Key Property | Benefit for Regulated Industries |
|---|---|
| FDA Approval | Certified safe for direct contact with food, drugs, and cosmetics. |
| Chemical Inertness | Will not react with or contaminate sensitive products. |
| Non-Porous Surface | Inhibits microbial growth and simplifies sterilization. |
| High-Temperature Resilience | Maintains integrity in demanding processing environments. |
| Non-Toxicity & Biocompatibility | Ideal for medical devices and pharmaceutical manufacturing. |
Ensure your components meet the highest standards of purity and safety.
KINTEK specializes in the precision manufacturing of FDA-approved virgin PTFE components—including seals, gaskets, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise in custom fabrication, from prototypes to high-volume orders, guarantees that your products deliver uncompromising performance and compliance.
Contact KINTEK today to discuss your specific application needs and receive a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- How do the costs of Teflon encapsulated O-rings compare to other sealing solutions? A Guide to Total Cost of Ownership
- What are the common applications of PTFE diaphragms in the Chinese market? Key Uses in Chemical, Pharma & Semiconductor
- How should designs account for Teflon's high creep rate? Master PTFE Design for Long-Term Reliability
- What are the two main types of additives in PTFE-based materials? Reinforcements vs. Fillers Explained
- When are split PTFE backup rings particularly suitable? Ideal for Retrofitting and High-Pressure Seals
- What are PTFE compounds and how are they used? Enhance Performance for Demanding Applications
- What is the purpose of sintering in the PTFE bush manufacturing process? | Achieve Superior Component Performance
- What customization options are available for PTFE wear strips and bands? Tailor Performance with Material, Size & Format



















