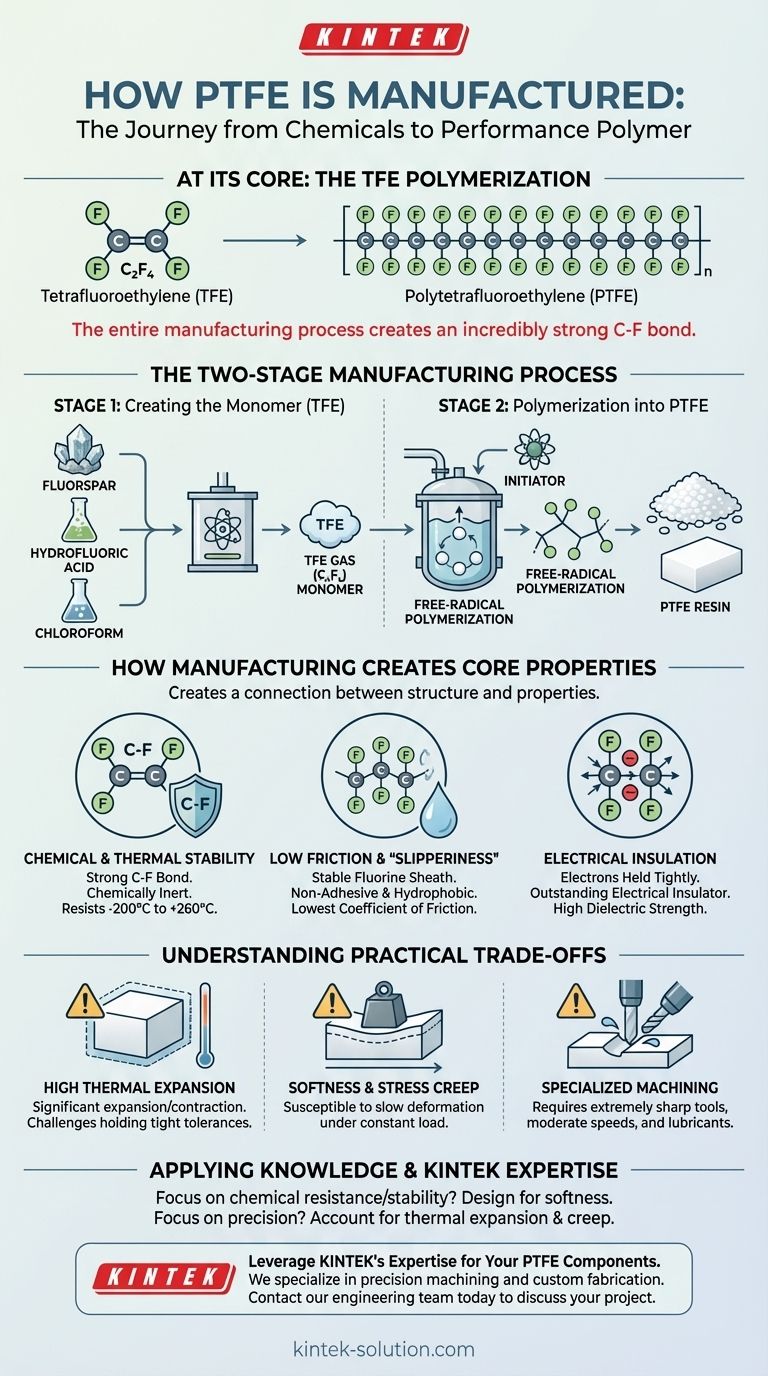
At its core, PTFE is manufactured through the polymerization of tetrafluoroethylene (TFE). This process begins by creating the TFE monomer gas from common industrial chemicals—fluorspar, hydrofluoric acid, and chloroform. This gas is then polymerized under specific conditions to form the stable, long-chain polymer we know as Polytetrafluoroethylene, or PTFE.
The entire manufacturing process is designed to create an incredibly strong and stable bond between carbon and fluorine atoms. This simple but powerful molecular structure is the direct source of all of PTFE’s famous properties: extreme chemical resistance, a wide temperature range, and the lowest coefficient of friction of any solid material.

The Two-Stage Manufacturing Process
The creation of PTFE is best understood as a two-stage chemical process. First, the basic building block must be created. Second, those building blocks are linked together to form the final material.
Stage 1: Creating the Monomer (TFE)
The journey to PTFE begins with the synthesis of its monomer, tetrafluoroethylene (C₂F₄). This gas is the essential precursor to the final polymer.
The raw ingredients for this stage are typically fluorspar, hydrofluoric acid, and chloroform. Through a series of chemical reactions, these materials are used to produce the TFE gas. This monomer is the fundamental unit that will be repeated to build the PTFE chain.
Stage 2: Polymerization into PTFE
Once the TFE monomer is synthesized, the crucial step of polymerization begins. This is the process of linking many individual monomer molecules into extremely long chains.
This is achieved through a free-radical polymerization process. An initiator is introduced to the TFE, which breaks the double bond in the TFE molecule and starts a chain reaction. Monomers rapidly add to the chain, resulting in the final product: a stable, white, waxy solid known as PTFE.
How Manufacturing Creates PTFE's Core Properties
The unique characteristics of PTFE are not accidental; they are a direct result of its molecular structure, which is locked in during manufacturing. The bond between carbon and fluorine is exceptionally strong, and the fluorine atoms form a protective sheath around the carbon backbone.
The Source of Chemical and Thermal Stability
The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry. This makes the molecule incredibly difficult to break apart with chemical or thermal energy.
This stability is why PTFE is chemically inert, resisting nearly all industrial chemicals and solvents. It’s also why it can withstand a vast temperature range, from –200°C to +260°C, without degrading.
The Origin of its "Slipperiness"
The fluorine atoms that encase the carbon chain are very stable and electrically balanced. They create a surface with extremely low intermolecular forces.
Because other substances have nothing to latch onto, the material becomes non-adhesive and hydrophobic (water-repelling). This same principle gives PTFE the lowest coefficient of friction of any known solid material.
The Reason for its Electrical Insulation
The electrons within the C-F bonds are held very tightly. This structure prevents the free flow of electrons through the material.
As a result, PTFE is an outstanding electrical insulator with high dielectric strength, making it an ideal choice for wire insulation and high-frequency electronic applications.
Understanding the Practical Trade-offs
While its properties are remarkable, they also introduce specific challenges, particularly when machining or designing high-precision parts. Understanding these trade-offs is critical for successful application.
High Thermal Expansion
The weak forces between the polymer chains, which contribute to its low friction, also mean the material expands and contracts significantly with temperature changes.
This high coefficient of thermal expansion can make holding tight tolerances a challenge. Parts must be designed and machined with their final operating temperature in mind to ensure dimensional accuracy.
Softness and Stress Creep
PTFE is a relatively soft material, which makes it easy to cut. However, this softness also means it is susceptible to stress creep—the tendency to slowly deform over time when under a constant load.
This requires careful management of clamping pressures during machining to avoid compressing the material and a design that accounts for potential long-term deformation.
The Need for Specialized Machining
Working with PTFE effectively means adapting to its nature. Forcing it into shape with incorrect methods will lead to poor results.
Success requires using extremely sharp, polished cutting tools (HSS or carbide), employing moderate cutting speeds to avoid heat buildup, and using lubricants to ensure a clean cut without melting or deforming the material.
Applying This Knowledge to Your Project
Understanding how PTFE is made provides direct insight into how it should be used. Your primary goal will determine your primary considerations.
- If your primary focus is chemical resistance and thermal stability: PTFE is an unparalleled choice for seals, gaskets, and linings in harsh chemical or high-temperature environments, but be sure your design can accommodate its mechanical softness.
- If your primary focus is achieving high-precision components: Success demands designing specifically for PTFE's properties by accounting for its high thermal expansion and potential for creep, and by specifying the correct machining protocols.
Ultimately, appreciating that PTFE's strengths and weaknesses originate from its powerful carbon-fluorine bond is the key to leveraging it effectively.
Summary Table:
| PTFE Manufacturing Stage | Key Inputs | Key Process | Key Output |
|---|---|---|---|
| Stage 1: Monomer Synthesis | Fluorspar, Hydrofluoric Acid, Chloroform | Chemical Reaction | Tetrafluoroethylene (TFE) Gas |
| Stage 2: Polymerization | TFE Monomer | Free-Radical Polymerization | Polytetrafluoroethylene (PTFE) Resin |
Leverage KINTEK's Expertise for Your PTFE Components
Understanding the science behind PTFE is the first step. Applying it effectively requires a manufacturing partner who masters the material's unique properties. KINTEK specializes in the precision machining and custom fabrication of high-performance PTFE components—from seals and liners to complex labware.
We serve the semiconductor, medical, laboratory, and industrial sectors, ensuring your parts are designed and produced to account for PTFE's thermal expansion and stress creep, guaranteeing optimal performance and longevity.
Ready to turn PTFE's potential into a precision solution for your application? Contact our engineering team today to discuss your project, from prototype to high-volume production.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
People Also Ask
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability



















