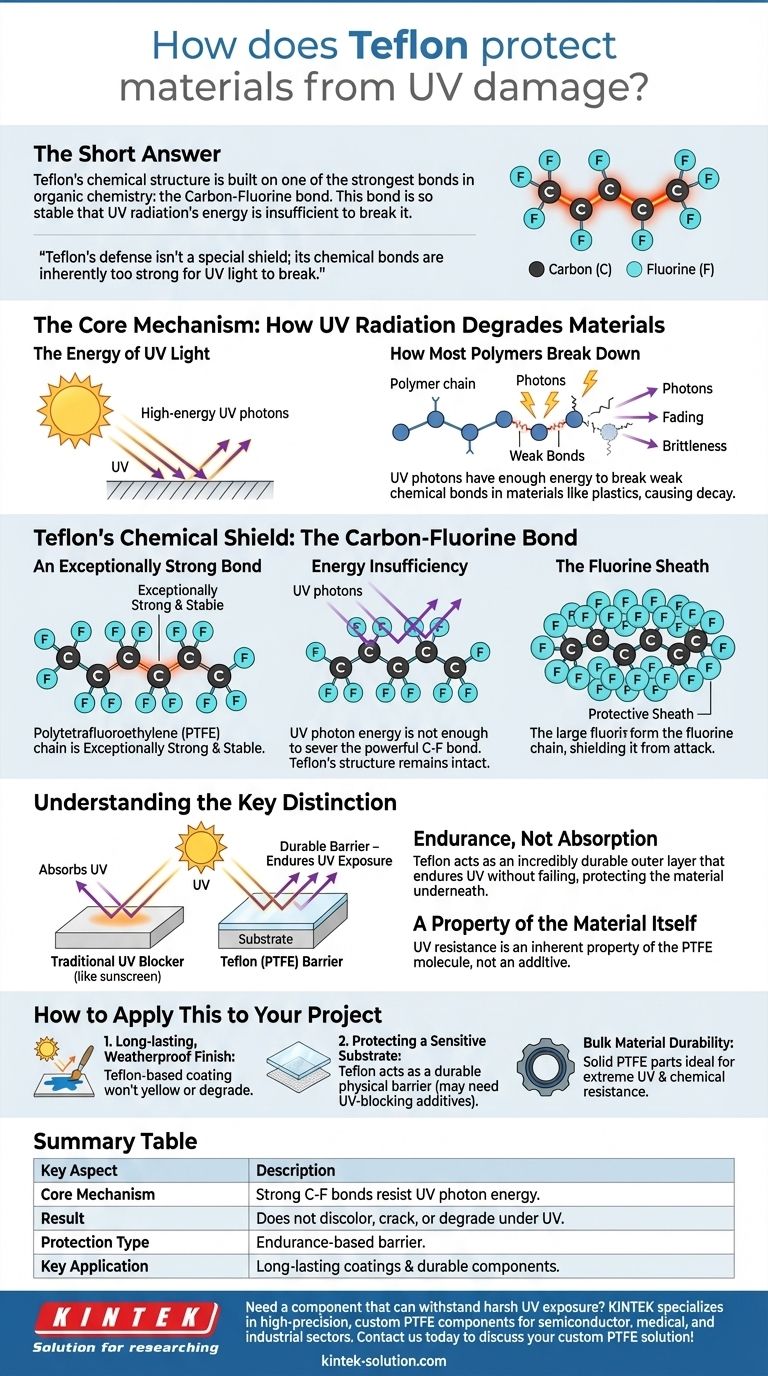
The short answer is that Teflon protects materials from UV damage because its chemical structure is built on one of the strongest bonds in organic chemistry: the Carbon-Fluorine bond. This bond is so stable that the energy from ultraviolet radiation is simply not powerful enough to break it apart. As a result, Teflon doesn't discolor, crack, or degrade under sun exposure.
The core problem with UV radiation is that its high energy shatters the chemical bonds of most materials, causing them to decay. Teflon's defense isn't a special shield or additive; its own chemical bonds are inherently too strong for UV light to break, making it exceptionally durable by nature.

The Core Mechanism: How UV Radiation Degrades Materials
To understand why Teflon is so resilient, we must first understand how UV light damages other materials. This process is known as photodegradation.
The Energy of UV Light
Sunlight contains ultraviolet (UV) radiation, which consists of high-energy photons. When these photons strike a surface, they transfer their energy to the molecules of the material.
How Most Polymers Break Down
In many common materials like plastics, paints, and fabrics, the chemical bonds holding the polymer chains together are relatively weak. The energy delivered by UV photons is sufficient to break these bonds, causing the material to lose its integrity. This manifests as fading, yellowing, brittleness, and eventual failure.
Teflon's Chemical Shield: The Carbon-Fluorine Bond
Teflon, or Polytetrafluoroethylene (PTFE), is fundamentally different from these other materials at a molecular level, which is the source of its legendary resilience.
An Exceptionally Strong Bond
The backbone of the Teflon polymer is made of carbon atoms, but each carbon atom is bonded to two fluorine atoms. The Carbon-Fluorine (C-F) bond is exceptionally strong and stable.
Energy Insufficiency
The energy contained within UV photons is simply not enough to sever the powerful C-F bond. While other materials absorb this energy and break apart, Teflon's structure remains intact and unaffected. It endures the bombardment without degrading.
The Fluorine Sheath
The fluorine atoms are also larger than hydrogen atoms (which are found in many other plastics). They effectively form a tight, protective "sheath" around the central carbon chain, further shielding it from chemical attack and environmental stress.
Understanding the Key Distinction
It's critical to distinguish how Teflon protects a surface versus how a traditional UV blocker works. This is a common point of confusion.
Endurance, Not Absorption
Teflon does not work like a sunscreen that absorbs or reflects UV rays. Instead, it protects by being an incredibly durable outer layer. It creates a physical barrier that endures UV exposure without failing, thereby protecting the material underneath it.
A Property of the Material Itself
This UV resistance is not an additive; it is an inherent property of the PTFE molecule. That is why Teflon is used as a coating (like in paint or on cookware) or as a solid material itself (like in seals and gaskets for outdoor equipment). The protection comes from the presence of Teflon itself.
How to Apply This to Your Project
Your choice of material should be guided by your specific goal and the nature of the UV threat you are trying to mitigate.
- If your primary focus is a long-lasting, weatherproof finish: A Teflon-based paint or coating is an excellent choice because the coating itself will not yellow, chalk, or degrade from sun exposure.
- If your primary focus is protecting a sensitive substrate: Teflon acts as a durable physical barrier. The coating will last for years, but standard Teflon is transparent to UV; the UV rays will still pass through and can affect the material beneath unless the formulation includes specific UV-blocking additives.
- If your primary focus is bulk material durability: Using solid PTFE components is ideal for parts that require extreme chemical and UV resistance, such as seals or insulators in outdoor or marine environments.
Ultimately, leveraging Teflon's properties correctly means understanding that its strength comes from its unwavering chemical stability.
Summary Table:
| Key Aspect | Description |
|---|---|
| Core Mechanism | Strong C-F bonds resist UV photon energy, preventing bond breakage. |
| Result | Teflon does not discolor, crack, or degrade under UV exposure. |
| Protection Type | Endurance-based barrier, not UV absorption or reflection. |
| Key Application | Ideal for long-lasting coatings and durable components in harsh environments. |
Need a component that can withstand harsh UV exposure? KINTEK specializes in manufacturing high-precision, custom PTFE components like seals, liners, and labware. Our PTFE parts leverage this inherent UV resistance to provide long-lasting performance in the semiconductor, medical, laboratory, and industrial sectors. From prototypes to high-volume orders, we deliver the durability your project requires.
Contact us today to discuss your custom PTFE solution!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications



















