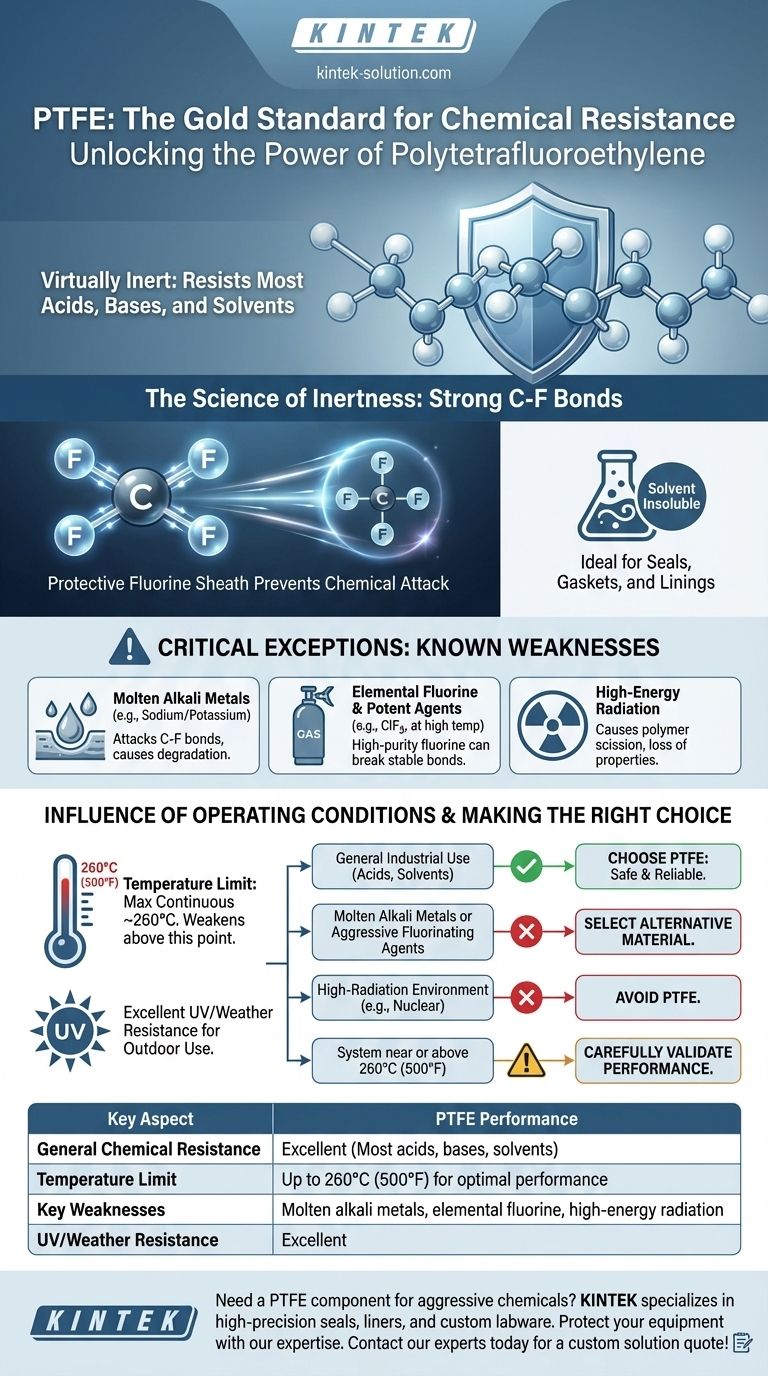
In short, Polytetrafluoroethylene (PTFE) offers exceptional chemical resistance, making it one of the most inert and reliable polymers available. It is virtually unaffected by the vast majority of industrial chemicals, acids, bases, and solvents. However, its resistance is not absolute and fails when exposed to a very specific class of highly reactive substances, particularly at elevated temperatures.
The core of PTFE's performance lies in its stable carbon-fluorine bonds, which render it chemically inert to almost everything. The critical takeaway is that while it is a default choice for chemical compatibility, you must verify that your application does not involve its few known weaknesses: molten alkali metals, elemental fluorine, or high-energy radiation.

The Foundation of PTFE's Chemical Inertness
PTFE's remarkable chemical resistance is not a surface-level property; it is inherent to its molecular structure. Understanding this structure is key to trusting its performance in your application.
The Power of the Carbon-Fluorine Bond
The PTFE molecule consists of a long chain of carbon atoms, where each carbon is completely sheathed by fluorine atoms. The bond between carbon and fluorine is exceptionally strong and stable.
This fluorine sheath effectively protects the vulnerable carbon backbone from chemical attack, rendering the entire polymer non-reactive and essentially inert.
Resistance to Solvents
A practical consequence of this inertness is PTFE's extreme insolubility. At room temperature, there are no known solvents that can dissolve PTFE.
This makes it an ideal material for seals, gaskets, and linings in systems handling a wide variety of fluid mixtures and aggressive solvents without risk of degradation.
Understanding the Critical Exceptions
While nearly universal, PTFE's chemical resistance has clear and well-documented limitations. Acknowledging these exceptions is crucial for safe and effective material selection.
Molten Alkali Metals
Substances like molten or dissolved sodium and potassium are among the few that can attack PTFE. These highly reactive metals can disrupt the carbon-fluorine bond, causing the material to degrade.
Elemental Fluorine and Potent Fluorinating Agents
It is logical that the element used to create PTFE's stability can also be its weakness. High-purity elemental fluorine (especially turbulent liquid or gas) and potent compounds like chlorine trifluoride (ClF3) can attack PTFE, particularly at elevated temperatures.
These aggressive agents are powerful enough to break the C-F bonds that give the material its inertness.
High-Energy Radiation
PTFE's primary non-chemical weakness is its poor resistance to high-energy radiation, such as gamma rays. This type of radiation can cause scission in the polymer chains, breaking the molecule down and leading to a rapid loss of mechanical properties.
The Influence of Operating Conditions
Chemical resistance is not just about the substance but also the environment. Temperature is the most significant factor that can influence PTFE's performance.
The Role of Temperature
PTFE maintains its elite chemical resistance up to its maximum continuous operating temperature of approximately 260°C (500°F).
Beyond this temperature, the material's structural integrity begins to weaken, making it more susceptible to attack by chemicals that it would easily resist at lower temperatures. Performance is considered optimal at or below 200°C.
UV and Weather Resistance
Complementing its chemical stability, PTFE is highly resistant to UV radiation and general weathering. This durability makes it a reliable choice for outdoor applications where it may be exposed to both environmental factors and chemical agents simultaneously.
Making the Right Choice for Your Application
Use these guidelines to determine if PTFE is the correct material for your specific needs.
- If your primary focus is general industrial use with common acids, solvents, or corrosive media: PTFE is an exceptionally safe and reliable choice, often considered the gold standard.
- If your application involves molten alkali metals or aggressive fluorinating agents: You must select an alternative material, as PTFE is known to fail under these specific conditions.
- If you are operating in a high-radiation environment (e.g., nuclear): Avoid PTFE, as radiation will cause it to degrade and lose its structural integrity.
- If your system operates consistently near or above 260°C (500°F): You must carefully validate PTFE's performance, as its chemical resistance can be compromised at these elevated temperatures.
By understanding both its profound inertness and its precise limitations, you can confidently deploy PTFE in the most demanding chemical environments.
Summary Table:
| Key Aspect | PTFE Performance |
|---|---|
| General Chemical Resistance | Excellent - Resists most acids, bases, and solvents |
| Temperature Limit | Up to 260°C (500°F) for optimal performance |
| Key Weaknesses | Molten alkali metals, elemental fluorine, high-energy radiation |
| UV/Weather Resistance | Excellent |
Need a PTFE component that can withstand your most aggressive chemicals?
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment is protected from corrosive media, enhancing safety and longevity.
We offer custom fabrication from prototypes to high-volume orders, tailoring solutions to your exact specifications.
Contact our experts today to discuss your chemical resistance requirements and get a custom solution quote!
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance



















