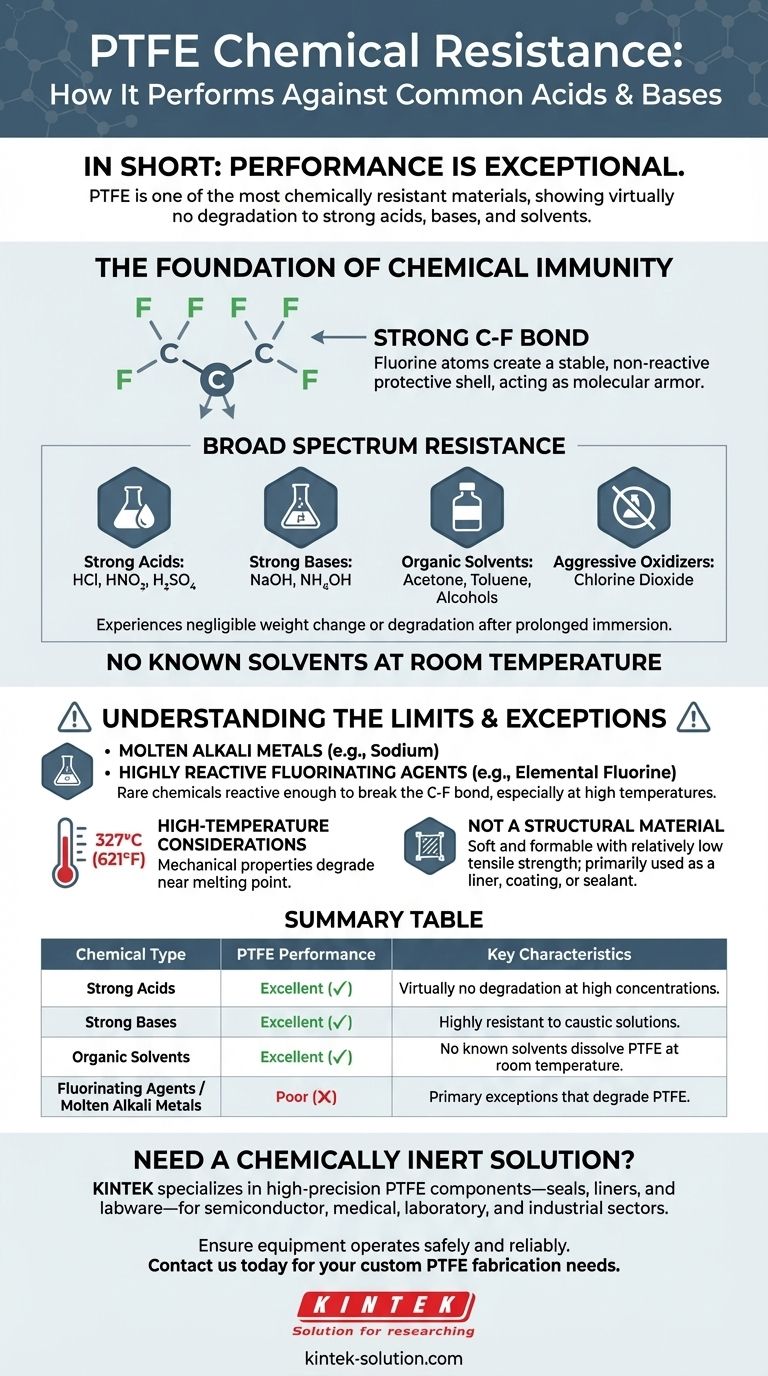
In short, the performance of PTFE against common acids and bases is exceptional. It is one of the most chemically resistant materials known, showing virtually no degradation when exposed to substances like hydrochloric acid, nitric acid, sulfuric acid, sodium hydroxide, and ammonium hydroxide, even at various concentrations and elevated temperatures.
The core reason for PTFE's unmatched chemical resistance is not just a feature, but a fundamental property of its molecular structure. The incredibly strong carbon-fluorine bonds make the material almost entirely inert, rendering it a default choice for the most demanding chemical environments.

The Foundation of PTFE's Chemical Immunity
To understand why PTFE (polytetrafluoroethylene) is so reliable, we must look at its chemistry. Its performance is not a coincidence but a direct result of its unique molecular composition.
The Strength of the Carbon-Fluorine Bond
At the heart of PTFE's inertness is the carbon-fluorine (C-F) bond. This is one of the strongest single bonds in organic chemistry.
The fluorine atoms create a tight, stable, and non-reactive protective shell around the carbon backbone of the polymer. This molecular armor prevents other chemicals from getting close enough to react.
Broad Spectrum Resistance
PTFE’s stability gives it an impressively wide range of chemical resistance. It is virtually unaffected by almost all industrial chemicals.
This includes:
- Strong Acids: Sulfuric acid, nitric acid, hydrochloric acid.
- Strong Bases: Sodium hydroxide, ammonium hydroxide.
- Organic Solvents: Acetone, toluene, benzene, and alcohols.
- Aggressive Oxidizers: Including powerful cleaning agents like chlorine dioxide.
Evidence shows that PTFE experiences negligible weight change or degradation after prolonged immersion in these substances.
No Solvents at Room Temperature
A key indicator of its resistance is that there are no known solvents capable of dissolving PTFE at or near room temperature. This makes it an invaluable material for containing and processing a vast array of reactive compounds.
Understanding the Limits and Exceptions
While PTFE is remarkably robust, no material is without its limits. Acknowledging its few specific vulnerabilities is critical for making an informed engineering decision.
The Few True Vulnerabilities
PTFE's chemical armor can be breached by a very small and specific group of substances.
The most notable exceptions are molten alkali metals (like sodium) and highly reactive fluorinating agents, such as elemental fluorine itself, particularly at high temperatures and pressures. These are the rare chemicals that are reactive enough to break the C-F bond.
High-Temperature Considerations
PTFE performs well at elevated temperatures, but its mechanical properties will degrade as it approaches its melting point of around 327°C (621°F).
When evaluating PTFE for high-temperature service in a corrosive environment, you must consider the combined effect of thermal stress and chemical exposure.
Not a High-Strength Structural Material
It is important to remember that PTFE is a soft and formable material with relatively low tensile strength. Its primary value is in its inertness and low-friction properties, not its load-bearing capacity.
Therefore, it is most often used as a liner, a coating, a sealant, or for components like tubing and containers rather than as a primary structural element.
How to Apply This to Your Project
Your specific goal will determine if PTFE is the optimal choice.
- If your primary focus is containing strong acids, bases, or organic solvents: PTFE is one of the most reliable and inert materials you can select for the application.
- If your primary focus is ensuring sample purity and preventing contamination: PTFE's non-reactive surface is ideal, as it will not leach or react with the contained chemical.
- If your environment involves molten alkali metals or elemental fluorine: You must seek an alternative material, as these are the few known substances that can attack PTFE.
Ultimately, for nearly all applications involving aggressive chemical exposure, PTFE provides a level of security and performance that few other polymers can match.
Summary Table:
| Chemical Type | PTFE Performance | Key Characteristics |
|---|---|---|
| Strong Acids (e.g., HCl, H₂SO₄) | Excellent | Virtually no degradation, even at high concentrations and temperatures. |
| Strong Bases (e.g., NaOH) | Excellent | Highly resistant to caustic solutions like sodium hydroxide. |
| Organic Solvents (e.g., Acetone, Toluene) | Excellent | No known solvents dissolve PTFE at room temperature. |
| Fluorinating Agents / Molten Alkali Metals | Poor | These are the primary exceptions that can degrade PTFE. |
Need a chemically inert solution for your application?
KINTEK specializes in manufacturing high-precision PTFE components—including seals, liners, and custom labware—for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment operates safely and reliably in the most aggressive chemical environments.
Contact us today to discuss your custom PTFE fabrication needs, from prototypes to high-volume orders.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications
- What are some exceptional properties of PTFE? Unlock Unmatched Performance in Extreme Environments
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- Why is chemical compatibility important when choosing a PTFE-coated septum? Avoid Sample Contamination and Data Loss



















