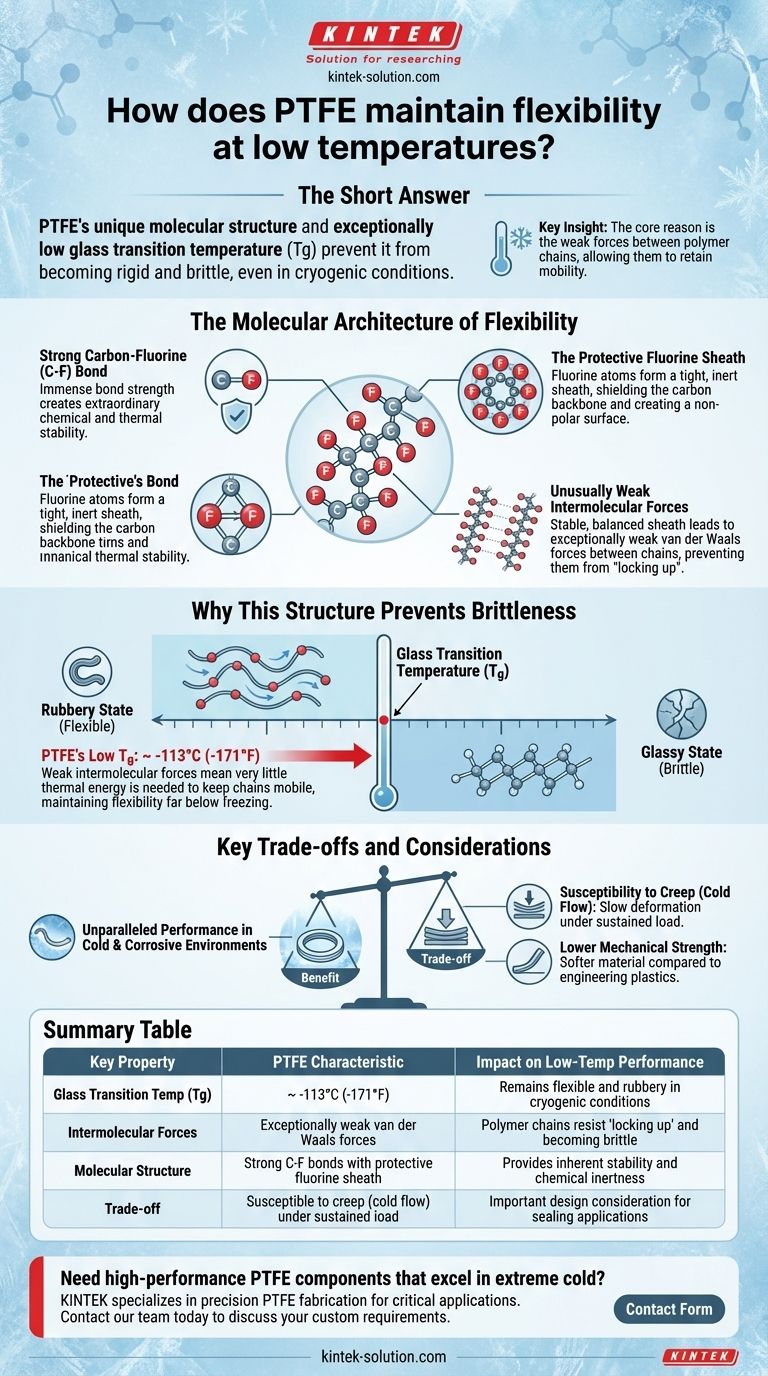
The short answer is that Polytetrafluoroethylene (PTFE) maintains its flexibility at extremely low temperatures due to its unique molecular structure. The strong carbon-fluorine bonds and the way the fluorine atoms wrap around the carbon backbone create a stable, low-friction polymer chain that resists becoming rigid and brittle, even in cryogenic conditions.
The core reason for PTFE's low-temperature performance is not just its chemical stability, but its exceptionally low glass transition temperature. This is a direct result of weak forces between its polymer chains, allowing them to retain mobility when other materials would become frozen and brittle.

The Molecular Architecture of Flexibility
To understand why PTFE excels in the cold, we must look at its structure at the molecular level. Its properties are not accidental; they are a direct consequence of its specific chemical makeup.
The Power of the Carbon-Fluorine Bond
PTFE is a fluoropolymer, consisting of a long chain of carbon atoms completely surrounded by fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds known in organic chemistry.
This immense bond strength makes the molecule itself incredibly stable and resistant to being broken down by chemical, thermal, or environmental attacks.
The Protective Fluorine Sheath
The fluorine atoms are larger than the carbon atoms they are bonded to. As a result, they form a tight, helical sheath around the carbon backbone.
This sheath effectively shields the carbon chain from external factors. It also creates a very smooth, non-polar, and chemically inert surface at the molecular level.
Unusually Weak Intermolecular Forces
The critical factor for low-temperature flexibility is the interaction between the polymer chains. Because the fluorine sheath is so stable and electrically balanced, the forces of attraction between adjacent PTFE molecules (known as van der Waals forces) are exceptionally weak.
Other polymers have stronger intermolecular forces that cause them to "lock up" and become stiff as thermal energy is removed (i.e., as they get colder). PTFE's chains, however, do not attract each other strongly.
Why This Structure Prevents Brittleness
The weak forces between PTFE chains directly influence a critical material property: the glass transition temperature, which is the defining factor for flexibility in the cold.
Understanding Glass Transition Temperature (Tg)
Every polymer has a glass transition temperature (Tg). Above this temperature, the material is in a rubbery, flexible state where its long polymer chains have enough energy to move and slide past one another.
Below the Tg, the material enters a hard, "glassy" state. The polymer chains are effectively frozen in place, causing the material to become rigid and brittle, making it susceptible to cracking under stress.
PTFE's Exceptionally Low Tg
Because the forces between PTFE's polymer chains are so weak, it takes very little thermal energy to keep them mobile. This results in an extremely low glass transition temperature, typically around -113°C (-171°F).
This means PTFE remains in its flexible, "rubbery" state long after most other plastics have become brittle, allowing it to function effectively in cryogenic applications.
Key Trade-offs and Considerations
The same molecular properties that grant PTFE its remarkable low-temperature performance also introduce important limitations that must be considered in any design.
Susceptibility to Creep (Cold Flow)
The weak intermolecular forces mean that under a sustained mechanical load, PTFE's polymer chains can slowly slide past one another. This phenomenon is known as creep or cold flow.
This can lead to a gradual deformation of parts over time, which is a critical design consideration for structural or high-pressure sealing applications.
Lower Mechanical Strength
Compared to many other engineering plastics like PEEK or Nylon, PTFE is a relatively soft material with lower tensile strength and wear resistance. Its strength comes from its stability and low friction, not its hardness.
For applications requiring higher mechanical integrity, filled grades of PTFE (e.g., glass-filled or carbon-filled) are often used to improve strength and reduce creep.
Making the Right Choice for Your Application
Understanding the "why" behind PTFE's behavior allows you to apply it correctly.
- If your primary focus is performance in cryogenic or extreme cold: PTFE is an exceptional choice for seals, gaskets, and flexible conduits where maintaining pliability is essential.
- If your application involves high mechanical load or pressure: You must account for PTFE's tendency to creep; consider using reinforced grades or alternative materials if the load is too high.
- If your main concern is chemical resistance: PTFE's inertness is world-class, but remember this property is directly linked to the same structure that makes it mechanically softer.
Ultimately, PTFE's molecular design makes it a specialist material, offering unparalleled performance in cold and corrosive environments by trading raw mechanical strength for chemical and thermal stability.
Summary Table:
| Key Property | PTFE Characteristic | Impact on Low-Temp Performance |
|---|---|---|
| Glass Transition Temp (Tg) | ~ -113°C (-171°F) | Remains flexible and rubbery in cryogenic conditions |
| Intermolecular Forces | Exceptionally weak van der Waals forces | Polymer chains resist 'locking up' and becoming brittle |
| Molecular Structure | Strong C-F bonds with a protective fluorine sheath | Provides inherent stability and chemical inertness |
| Trade-off | Susceptible to creep (cold flow) under sustained load | Important design consideration for sealing applications |
Need high-performance PTFE components that excel in extreme cold? KINTEK specializes in precision PTFE fabrication for semiconductor, medical, laboratory, and industrial applications. Our expertise ensures your seals, liners, and labware maintain critical flexibility and chemical resistance, even in cryogenic environments. Contact our team today to discuss your custom PTFE requirements, from prototypes to high-volume production.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers



















