In chemical-heavy environments, Nylon offers moderate resistance but has critical vulnerabilities. Its performance is highly dependent on the specific chemicals involved. While it holds up well against many common substances like oils and greases, it can be severely degraded by strong acids, alkalis (strong bases), and certain organic solvents.
Nylon is a mechanically robust material whose primary weakness is not universal chemical failure, but a specific susceptibility to strong acids, bases, and moisture. This hygroscopic nature is often the most significant factor, causing dimensional instability and a reduction in mechanical properties that must be accounted for in any design.

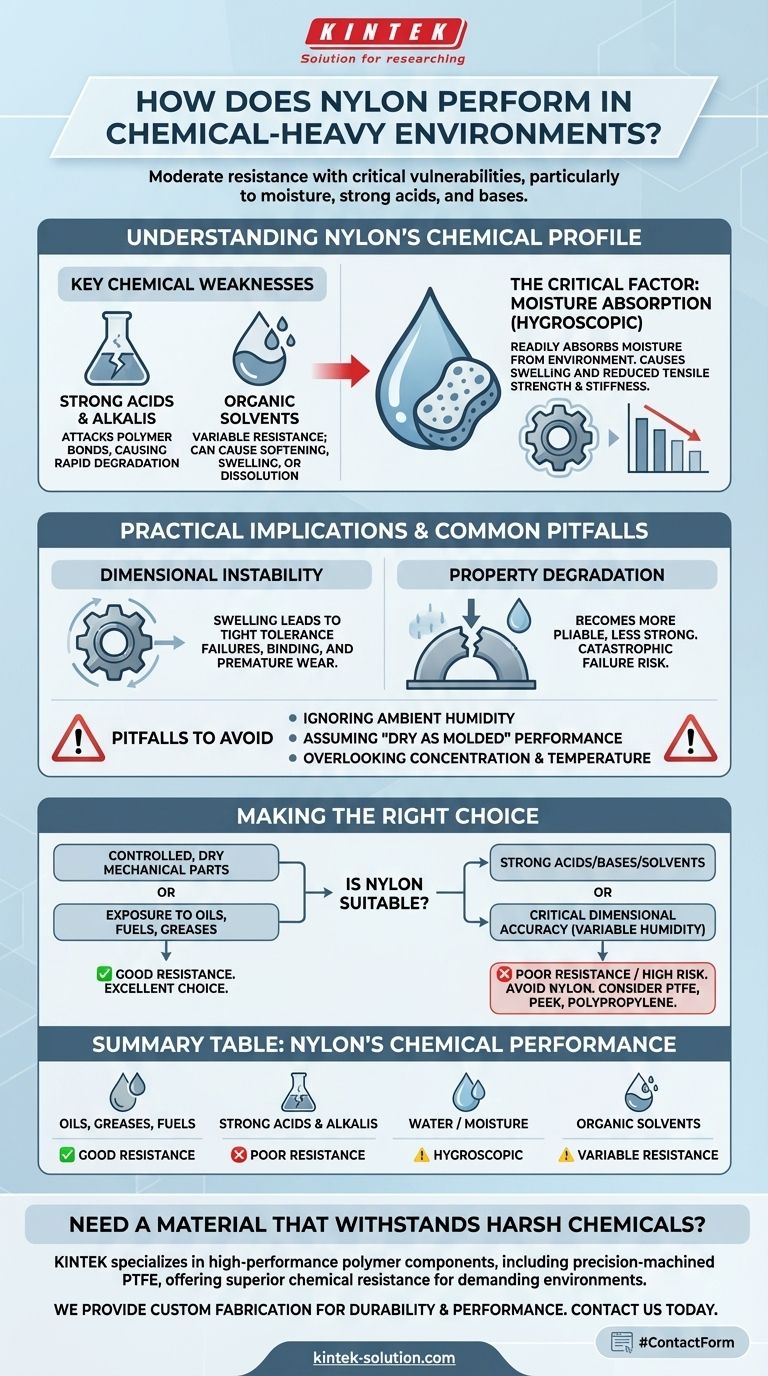
Understanding Nylon's Chemical Profile
To properly evaluate Nylon, we must move beyond a simple "good" or "bad" rating. Its interaction with chemicals is specific and predictable, with one particular element—water—playing a dominant role.
Key Chemical Weaknesses
Nylon's polymer structure is vulnerable to certain categories of chemicals. Strong acids and strong alkalis are particularly damaging because they attack and break down the amide bonds in the polymer chain, leading to a loss of material integrity and strength.
Exposure to certain organic solvents can also be problematic. While resistant to many, some can cause the material to soften, swell, or dissolve over time. Always consult a specific chemical compatibility chart for your exact grade of Nylon and the solvent in question.
The Critical Factor: Moisture Absorption
The most common and often overlooked chemical interaction for Nylon is with water. Nylon is hygroscopic, meaning it readily absorbs moisture from the surrounding environment, including ambient humidity.
This absorption has two major consequences. First, it causes the material to swell, changing its physical dimensions. Second, the water molecules act as a plasticizer, reducing tensile strength and stiffness while increasing impact strength and flexibility.
Practical Implications of Chemical Exposure
Understanding the theory is one thing; seeing its impact on a finished part is what truly matters for engineers and designers.
Dimensional Instability
For any application requiring tight tolerances, the swelling caused by moisture absorption can be a critical failure point. A precisely machined Nylon gear or bushing can go out of tolerance simply due to changes in environmental humidity, leading to binding or premature wear.
Degradation of Mechanical Properties
When Nylon absorbs moisture, it becomes more pliable and less strong. A part designed for a specific load-bearing capacity in a dry state may fail under the same load in a humid environment. Similarly, chemical attack from acids or bases directly weakens the material, risking catastrophic failure.
Common Pitfalls to Avoid
Many design failures involving Nylon stem from a few common oversights regarding its chemical properties.
Ignoring Environmental Humidity
Designers often vet Nylon against direct liquid chemical contact but forget that ambient air is a chemical environment. A part used in a coastal region or a tropical climate will have fundamentally different properties than the same part used in a desert.
Assuming "Dry" Part Performance
The mechanical properties listed on a technical data sheet are often for "dry as molded" material. Real-world performance will almost always be based on the "conditioned" state after the material has absorbed moisture and reached equilibrium with its environment.
Overlooking Concentration and Temperature
Chemical resistance is not a binary property. A material's ability to withstand a chemical often depends on the chemical's concentration and the operating temperature. Nylon might tolerate a cool, dilute acid but fail rapidly when exposed to a hot, concentrated version of the same chemical.
Making the Right Choice for Your Application
Use these guidelines to determine if Nylon is a suitable choice for your specific goal.
- If your primary focus is mechanical parts in a controlled, dry environment: Nylon is an excellent choice, valued for its strength, toughness, and wear resistance.
- If your application involves exposure to oils, fuels, or greases: Nylon generally performs very well and is a standard material for these scenarios.
- If your part will contact strong acids, bases, or specific aggressive solvents: You must avoid Nylon and consider more chemically inert polymers like polypropylene, PEEK, or PTFE.
- If dimensional accuracy is critical in a variable-humidity environment: The swelling caused by moisture absorption makes Nylon a high-risk choice unless these changes are specifically accounted for in the design.
Ultimately, understanding Nylon's specific vulnerabilities, especially to moisture, is the key to leveraging its strengths while avoiding material failure.
Summary Table:
| Chemical Environment | Nylon's Performance | Key Consideration |
|---|---|---|
| Oils, Greases, Fuels | ✅ Good Resistance | A standard choice for these applications. |
| Strong Acids & Alkalis | ❌ Poor Resistance | Attacks polymer bonds, causing rapid degradation. |
| Water / Moisture | ⚠️ Hygroscopic | Absorbs water, leading to swelling and reduced strength. |
| Organic Solvents | ⚠️ Variable Resistance | Performance depends on the specific solvent; check compatibility charts. |
Need a Material That Withstands Harsh Chemicals?
Nylon's vulnerabilities in aggressive environments can lead to part failure, downtime, and costly replacements. For applications involving strong acids, bases, or where dimensional stability is critical, a more chemically inert material is essential.
KINTEK specializes in high-performance polymer components, including precision-machined PTFE, which offers superior chemical resistance for the most demanding environments in the semiconductor, medical, laboratory, and industrial sectors.
We provide custom fabrication from prototypes to high-volume orders, ensuring your components meet exact specifications for durability and performance.
Contact us today to discuss your application requirements and find the right material solution.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Customizable PTFE Seals Filter Holders for Versatile Applications
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
People Also Ask
- In which industries is PTFE commonly used? Key Applications for Chemical & Thermal Resistance
- What are the common characteristics of Teflon? Unlocking Extreme Chemical and Thermal Resistance
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance



















