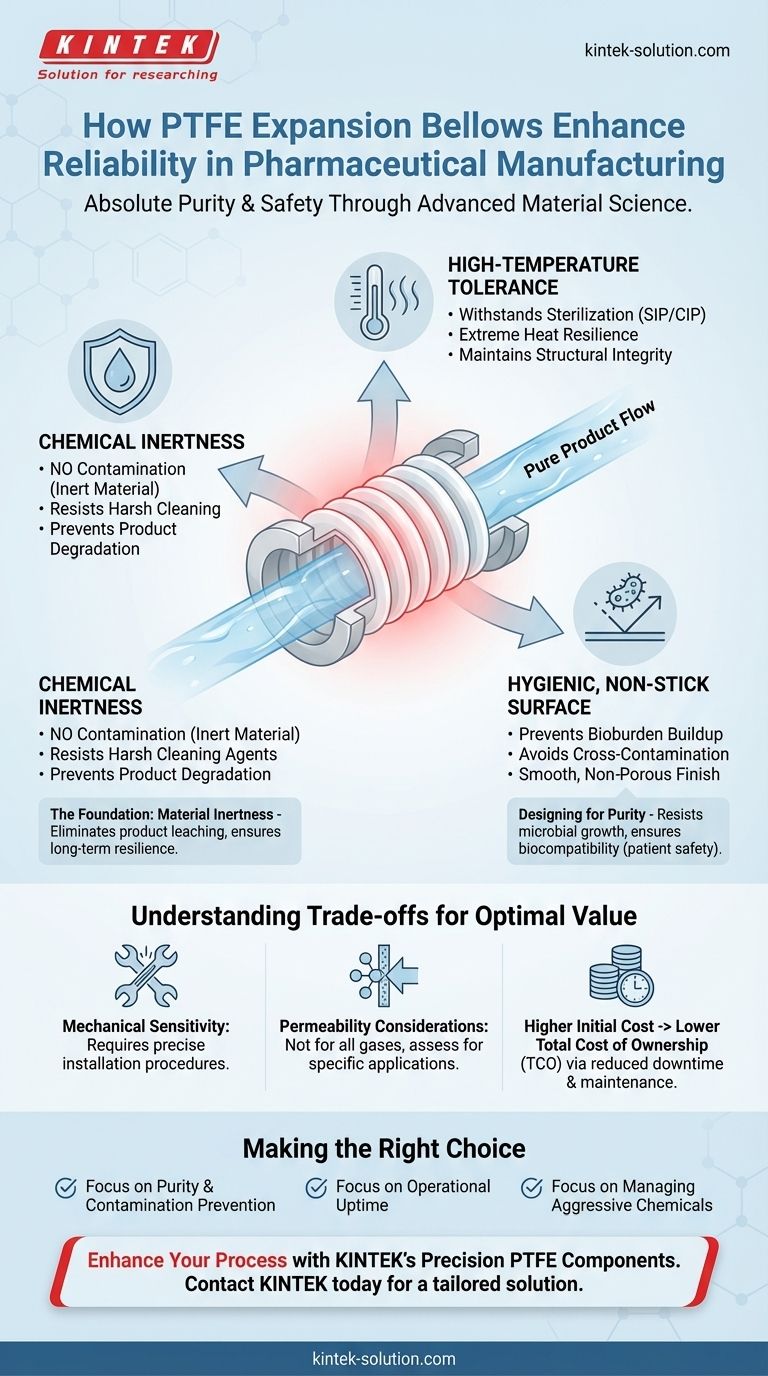
In pharmaceutical manufacturing, reliability is not just about uptime; it's about absolute purity and safety. PTFE expansion bellows directly enhance this reliability by providing exceptional chemical inertness, tolerating high-temperature sterilization, and offering a hygienic, non-stick surface. This powerful combination prevents product contamination, resists degradation from harsh cleaning agents, and ultimately reduces costly maintenance and operational downtime.
The core value of PTFE expansion bellows lies in their ability to create a chemically and biologically inert barrier within a processing system. This ensures the component itself never becomes a vector for contamination, whether through chemical reaction, material degradation, or microbial growth, thus guaranteeing product integrity and operational consistency.

The Foundation of Reliability: Material Inertness
The primary reason PTFE is specified for demanding pharmaceutical applications is its profound lack of reactivity. This single property solves multiple critical challenges simultaneously.
Eliminating Product Contamination
PTFE is one of the most chemically inert materials known. It will not react with, leach into, or otherwise contaminate the sensitive and valuable compounds being manufactured.
This non-reactive nature ensures the final drug product remains pure, meeting the stringent regulatory requirements for patient safety.
Resisting Aggressive Cleaning Agents
Pharmaceutical equipment undergoes rigorous cleaning-in-place (CIP) and sterilization-in-place (SIP) cycles involving harsh chemicals and high temperatures.
PTFE bellows withstand these aggressive cleaning agents without degrading. This resilience prevents component failure and ensures a long, predictable service life.
Tolerating Extreme Sterilization Temperatures
The ability to withstand high-temperature sterilization, such as with steam, is non-negotiable in sterile drug manufacturing.
PTFE maintains its structural integrity and performance characteristics even when exposed to extreme heat, ensuring the entire system can be reliably sterilized without compromising component integrity.
Designing for Purity and Preventing Bioburden
Beyond chemical inertness, the physical properties of PTFE are engineered to maintain a sterile processing environment. This focus on hygiene is critical for preventing contamination between batches.
The Importance of a Non-Stick Surface
PTFE has an exceptionally smooth, non-stick surface. This hygienic property prevents biological or chemical materials from building up on the component's walls.
This quality is essential for ensuring complete cleanability and preventing cross-contamination, which is paramount for product safety and quality.
Resisting Microbial Growth
The non-porous and smooth surface of PTFE provides no microscopic harbors for bacteria or other microbes to colonize.
This inherent resistance to microbial attachment makes the entire system easier to maintain in a sterile state, significantly reducing the risk of bioburden.
Ensuring Biocompatibility
In the unlikely event that any microscopic debris were to shed from a PTFE component, the material is biologically inert. This means it can pass through the body without consequence, adding a crucial layer of patient safety.
Understanding the Trade-offs
While PTFE offers exceptional performance, it's important to understand its specific characteristics to ensure proper application. No material is a universal solution.
Sensitivity to Mechanical Stress
Compared to metal alternatives, PTFE can be more susceptible to creep or damage from improper installation or excessive mechanical stress.
Following precise installation torque and alignment procedures is critical to maximizing the service life of PTFE bellows and preventing premature failure.
Permeability Considerations
While excellent for liquid barriers, PTFE is not completely impermeable to all gases. In highly specific applications involving sensitive gases, this property must be considered in the system design.
Higher Initial Cost
PTFE components typically have a higher upfront procurement cost than many standard elastomers. However, this initial investment is almost always offset by a lower total cost of ownership, driven by significantly reduced maintenance, less downtime, and mitigated contamination risk.
Making the Right Choice for Your System
Selecting the right material is a strategic decision that directly impacts product quality, patient safety, and operational efficiency.
- If your primary focus is product purity and preventing contamination: PTFE is the superior choice due to its unmatched chemical inertness and hygienic, non-stick surface.
- If your primary focus is operational uptime and reducing maintenance: The high cycle life and resistance to both sterilization and cleaning agents make PTFE a long-term, cost-effective solution.
- If your primary focus is managing aggressive or sensitive chemical compounds: PTFE's non-reactive nature ensures that neither your product nor the component will be degraded by chemical interaction.
Ultimately, selecting PTFE expansion bellows is a strategic decision to de-risk your manufacturing process, ensuring consistent quality, safety, and operational efficiency.
Summary Table:
| Key Benefit | Impact on Pharmaceutical Manufacturing |
|---|---|
| Chemical Inertness | Prevents product contamination and reacts with cleaning agents |
| High-Temperature Tolerance | Withstands sterilization cycles (SIP/CIP) without degradation |
| Non-Stick, Hygienic Surface | Reduces bioburden and prevents cross-contamination |
| Biocompatibility | Adds a critical layer of patient safety |
| Long Service Life | Lowers total cost of ownership by reducing maintenance and downtime |
Enhance the reliability and purity of your pharmaceutical manufacturing process with KINTEK's precision PTFE components.
Our PTFE expansion bellows, seals, liners, and labware are manufactured to the highest standards, providing the chemical inertness and hygienic properties essential for your critical applications. Whether you need a custom prototype or high-volume production for the semiconductor, medical, or laboratory industries, we prioritize precision to ensure your system's integrity.
Reduce contamination risk and operational downtime – Contact KINTEK today to discuss your specific requirements and receive a tailored solution.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
People Also Ask
- What are some applications of CNC machined PTFE parts? Critical Components for Medical, Electrical & Food Industries
- Why is dimensional stability a concern when machining PTFE? Ensure Accurate, Stable PTFE Components
- How is PTFE used in industrial processes? Maximize Safety and Efficiency
- What are some important physical property values for PTFE? Master Its Extreme Performance for Demanding Applications
- What is the working temperature range of PTFE? Master Extreme Heat and Cryogenic Applications



















