In practice, Polytetrafluoroethylene (PTFE) is one of the most chemically inert materials available. Its chemical resistance is nearly total across its operating temperature range, making it exceptionally stable when exposed to the vast majority of industrial chemicals, including highly corrosive acids, bases, and solvents. However, its near-invulnerability has a few, very specific exceptions.
While PTFE offers superior resistance to almost every chemical, its integrity is compromised by a select group of reactive agents. Understanding these rare but critical exceptions—namely molten alkali metals and potent fluorinating agents—is essential for its safe and effective use in demanding environments.

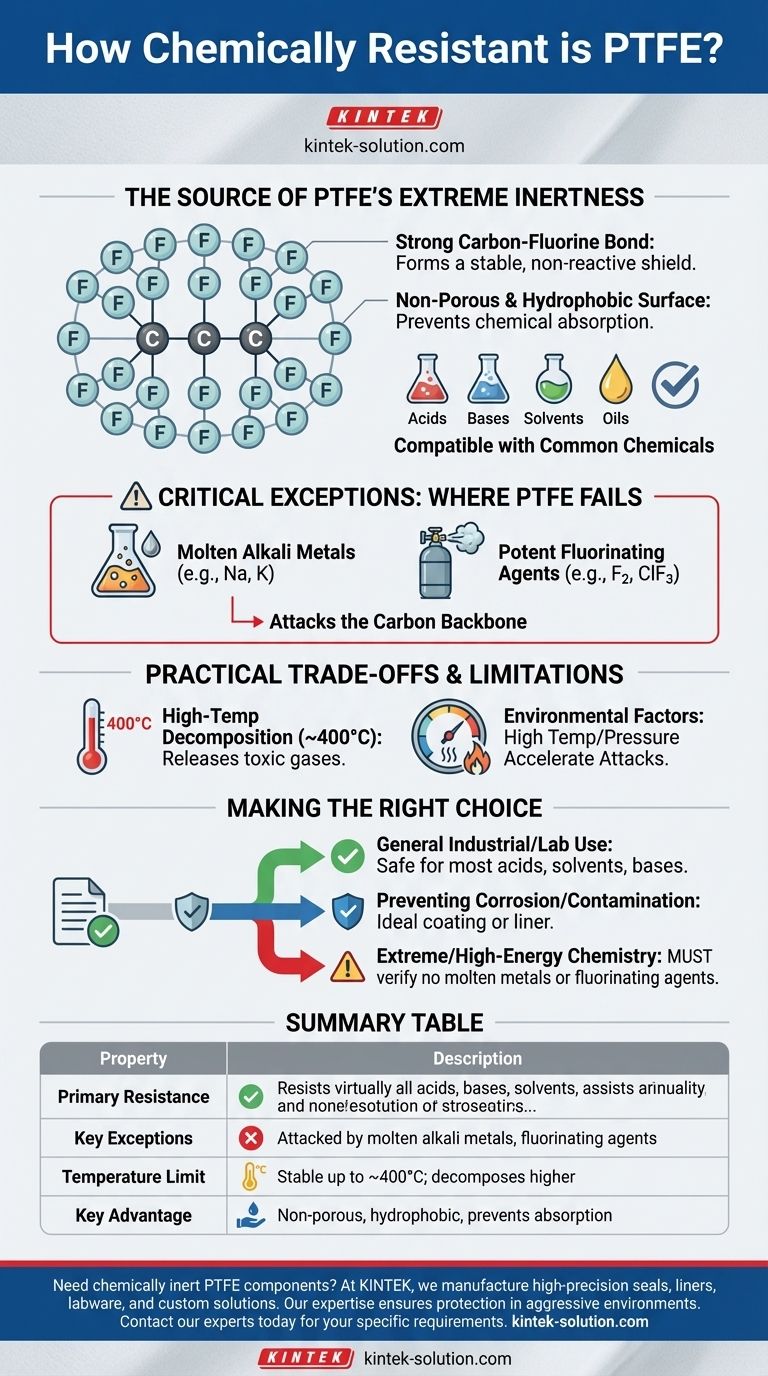
The Source of PTFE's Extreme Inertness
PTFE's remarkable chemical resistance is not an accident; it is a direct result of its unique molecular structure. This structure creates a material that is both physically and chemically non-reactive in most situations.
The Strength of the Carbon-Fluorine Bond
The foundation of PTFE's stability lies in the powerful bonds between its carbon and fluorine atoms. Fluorine atoms are highly electronegative and form an incredibly strong, stable bond with the carbon backbone of the polymer.
This arrangement creates a protective "sheath" of fluorine atoms around the carbon chain. This sheath effectively shields the carbon backbone from attack by outside chemical agents.
A Non-Reactive and Non-Porous Surface
PTFE is hydrophobic, meaning it does not absorb water. Furthermore, there are no known solvents that can dissolve it at room temperature.
This non-porous and non-absorbent nature prevents chemicals from penetrating the material, further enhancing its durability and inertness in diverse chemical environments.
Understanding the Scope of Chemical Resistance
While often described as universally resistant, a more precise understanding requires looking at which chemicals it resists and, more importantly, which ones it does not.
Compatibility with Common Chemicals
PTFE is an excellent choice for applications involving a wide array of chemical classes. It remains completely unaffected by continuous exposure to substances such as:
- Concentrated and dilute acids
- Alkalis and bases
- Alcohols and ketones
- Greases and oils
- Aromatic and halogenated hydrocarbons
The Critical Exceptions: Where PTFE Fails
Despite its incredible stability, PTFE is not completely immune to chemical attack. A few highly reactive substances are aggressive enough to break its strong carbon-fluorine bonds.
The primary exceptions are:
- Molten or Dissolved Alkali Metals: Liquid sodium and potassium are reactive enough to attack PTFE.
- Potent Fluorinating Agents: High-temperature gaseous fluorine, chlorine trifluoride, and oxygen difluoride can corrode the material.
These exceptions are rare in most industrial settings but are critical to identify when designing systems for extreme chemical service.
The Practical Trade-offs and Limitations
Chemical inertness is PTFE's defining feature, but it is not the only factor to consider in a real-world application. Its operational limits are defined by both chemical and thermal boundaries.
High-Temperature Decomposition
While stable across a wide service temperature range, PTFE will decompose at approximately 400°C (752°F). When it decomposes, it releases toxic and corrosive fluorinated gases.
This thermal limit is a critical safety consideration in any high-temperature application.
Environmental Conditions Matter
The reactivity of PTFE's few chemical exceptions is often magnified by environmental factors. High temperatures and high pressures can accelerate chemical attacks from substances like fluorine gas.
Therefore, evaluating compatibility requires assessing not just the chemical itself, but the full scope of the operating conditions.
Making the Right Choice for Your Goal
Selecting PTFE is about matching its unique profile to your specific operational needs. Its near-total inertness makes it a default choice for many, but a critical review is always necessary.
- If your primary focus is general industrial or laboratory use: PTFE is an exceptionally safe and reliable choice for handling the vast majority of common acids, solvents, and bases.
- If your primary focus is preventing corrosion and contamination: PTFE's non-reactive and non-stick surface makes it an ideal coating or liner for vessels, seals, and fluid handling systems.
- If you are working with extreme or high-energy chemistry: You must rigorously confirm that your process does not involve molten alkali metals or aggressive fluorinating agents, as these can cause material failure.
Ultimately, leveraging PTFE's power comes from respecting its well-defined limitations.
Summary Table:
| Property | Description |
|---|---|
| Primary Resistance | Resists virtually all acids, bases, solvents, oils, and hydrocarbons. |
| Key Exceptions | Attacked by molten alkali metals (e.g., sodium) and potent fluorinating agents (e.g., fluorine gas). |
| Temperature Limit | Stable up to ~400°C (752°F); decomposes at higher temperatures. |
| Key Advantage | Non-porous, hydrophobic, and insoluble, preventing chemical absorption and corrosion. |
Need chemically inert PTFE components for your application?
At KINTEK, we specialize in manufacturing high-precision PTFE seals, liners, labware, and custom components for the semiconductor, medical, laboratory, and industrial sectors. Our expertise ensures your equipment is protected from even the most aggressive chemicals, guaranteeing performance and preventing contamination.
Whether you require a standard part or a custom-fabricated solution from prototype to high-volume production, we deliver the precision and reliability you need.
Contact our experts today to discuss your specific chemical resistance requirements and find the perfect PTFE solution.
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Square Trays for Industrial and Laboratory Use
People Also Ask
- What are the unique properties of PTFE that make it commercially valuable? Unlock Unmatched Performance
- Why are PTFE vials considered environmentally friendly? Reduce Lab Waste with Durable Reusables
- What are the primary applications of PTFE? Unlocking High-Performance Solutions
- What material is the PTFE bottle made from? Discover the Benefits of 100% Virgin PTFE
- What makes the PTFE bottle durable? Unmatched Chemical & Thermal Stability for Demanding Applications



















