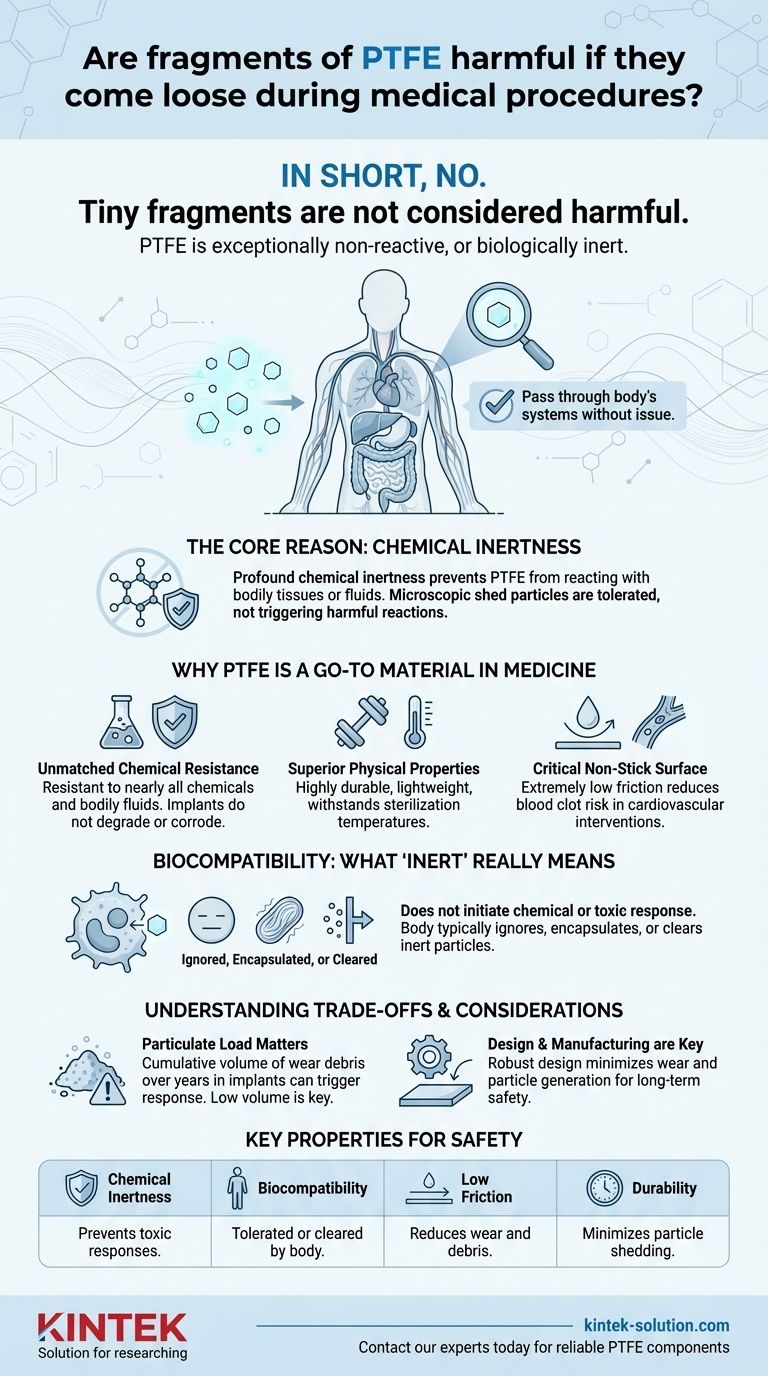
In short, no. Tiny fragments of Polytetrafluoroethylene (PTFE) that may come loose during the use of a medical device are not considered harmful. This is because PTFE is exceptionally non-reactive, or biologically inert. The material does not break down or trigger a toxic response, allowing these small particles to pass through the body's systems without issue.
The core reason for PTFE's safety in medical applications is its profound chemical inertness. This stability prevents it from reacting with bodily tissues or fluids, meaning that even if microscopic particles are shed, they are tolerated by the body rather than causing a harmful chemical or biological reaction.

Why PTFE is a Go-To Material in Medicine
The use of PTFE in medical devices is not accidental; it is a deliberate choice based on a unique combination of beneficial properties that ensure both performance and patient safety.
### Unmatched Chemical Resistance
PTFE is resistant to nearly all chemicals, acids, and bodily fluids. This ensures that a device component made from PTFE will not degrade or corrode over time when implanted in the human body.
### Superior Physical Properties
The material is highly durable, lightweight, and resistant to wear and tear. It can also withstand a wide range of temperatures, which is critical for sterilization processes before a medical procedure.
### Critical Non-Stick Surface
One of PTFE's most famous qualities is its extremely low coefficient of friction, creating a "non-stick" surface. In cardiovascular interventions like catheters or grafts, this ensures smoother passage and reduces the risk of blood clots forming on the device's surface.
The Principle of Biocompatibility: What "Inert" Really Means
The concept of being "inert" is central to understanding why loose PTFE particles are not a significant concern. It is the foundation of the material's biocompatibility.
### A Non-Reactive Barrier
A material is considered biologically inert when it does not initiate a chemical response or toxic effect when it comes into contact with living tissue. PTFE's molecular structure is incredibly stable, meaning it doesn't release chemicals or break down into harmful substances inside the body.
### How the Body Responds to Inert Particles
When the body encounters a foreign particle, its immune system typically investigates. Because PTFE particles are chemically non-reactive, they do not trigger an aggressive inflammatory or toxic reaction. Small, inert particles are typically either ignored, encapsulated by fibrous tissue, or cleared by the body's natural filtration systems.
Understanding the Trade-offs and Considerations
While individual PTFE microparticles are non-toxic, a complete technical assessment requires acknowledging the mechanical, rather than chemical, aspects of particulate debris in long-term implants.
### Particulate Load Matters
The primary concern in fields like joint replacement is not the toxicity of a single particle but the cumulative volume of wear debris over many years. A high volume of any inert particle—whether plastic, metal, or ceramic—can trigger a response where the body tries to clean up the debris, which can sometimes lead to inflammation or implant loosening over the long term.
### Design and Manufacturing are Key
This is why medical device engineering focuses intensely on minimizing wear and friction from the start. The goal is to design components, such as PTFE liners in joint replacements, that are so durable and smooth that the generation of wear particles is kept to an absolute minimum over the device's entire lifespan. The safety profile relies on both the material's inertness and the device's robust design.
Applying This to Your Perspective
Understanding the role of PTFE allows for a more informed assessment of medical device safety, whether you are a designer, clinician, or patient.
- If your primary focus is on material selection: PTFE remains a premier choice for applications requiring extreme chemical resistance and low friction, as its inertness provides a fundamental layer of safety.
- If your primary focus is on long-term device performance: The critical factor becomes the engineering design that minimizes wear and prevents the generation of particulate debris in the first place.
- If your primary focus is patient safety: You can be confident that the material itself is non-toxic, and regulatory standards for medical devices rigorously test for durability to ensure any particle shedding is negligible.
Ultimately, PTFE is trusted in medicine because its fundamental chemical stability provides a powerful and reliable foundation for safety.
Summary Table:
| Key Property | Why It Matters for Safety |
|---|---|
| Chemical Inertness | Does not react with bodily tissues or fluids, preventing toxic responses. |
| Biocompatibility | Non-reactive particles are tolerated or cleared by the body without harm. |
| Low Friction | Reduces wear and the generation of debris in the first place. |
| Durability | Withstands sterilization and long-term use, minimizing particle shedding. |
Need reliable, high-precision PTFE components for your medical devices?
At KINTEK, we specialize in manufacturing PTFE seals, liners, and custom labware for the medical, semiconductor, and laboratory industries. Our commitment to precision production and custom fabrication—from prototypes to high-volume orders—ensures your components meet the highest standards of safety and performance.
Contact our experts today to discuss how our PTFE solutions can enhance your product's reliability and patient safety.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
People Also Ask
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs



















