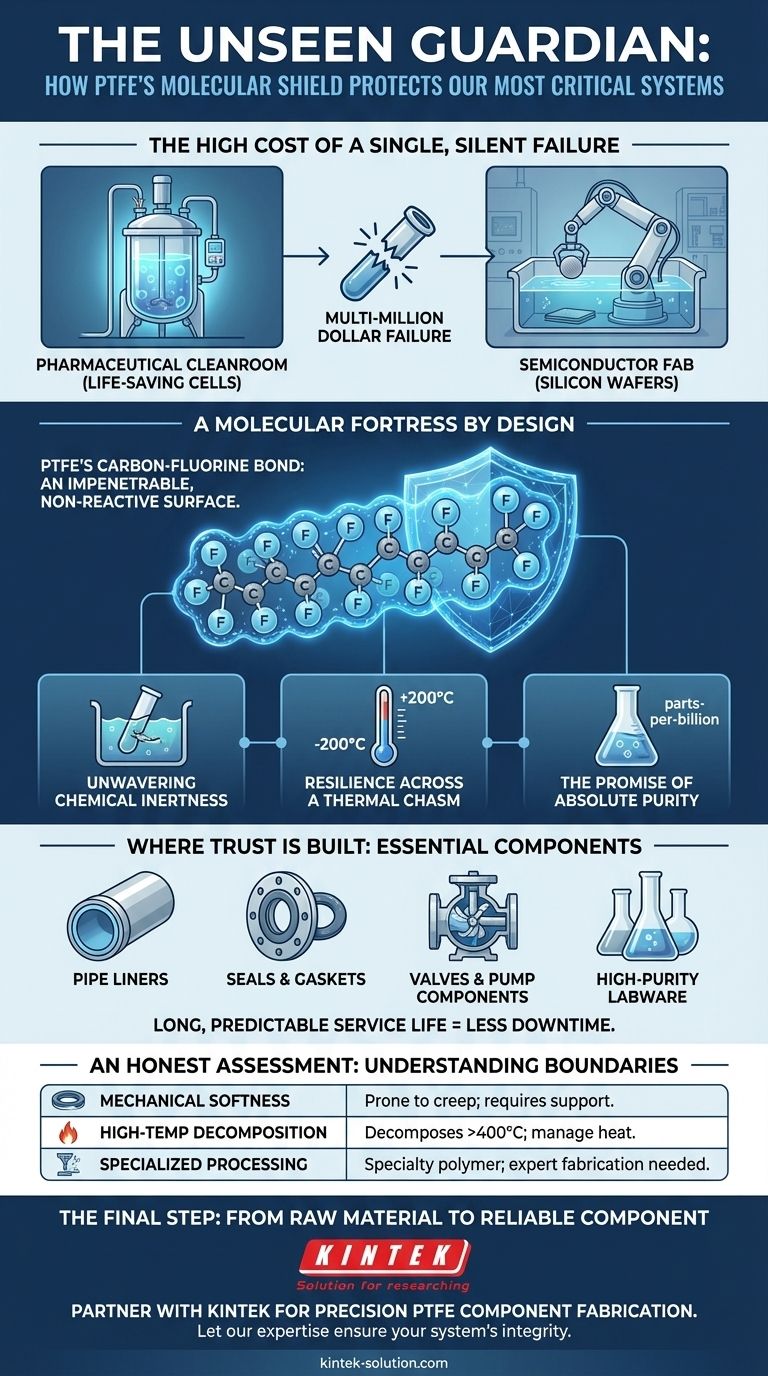
The High Cost of a Single, Silent Failure
In a pharmaceutical cleanroom, a bioreactor cultivates life-saving cells. In a semiconductor fab, a robotic arm bathes a silicon wafer in ultra-pure acid. In both scenarios, the operation is a success or a multi-million dollar failure based on something most people never see: the integrity of the tubes, seals, and vessels containing the process fluids.
The core challenge isn't just containing a chemical. It's ensuring the container does not react with, shed particles into, or degrade from the chemical it holds. It's a battle for absolute purity and predictability fought at a molecular level.
The Psychology of Material Choice: A Bet on Predictability
Engineers are professional pessimists. They design for failure. When they select a material for a critical application, they aren't just looking at its strengths; they're scrutinizing its potential for unexpected behavior.
This is why Polytetrafluoroethylene (PTFE) has become a cornerstone of high-stakes industries. Its selection is a psychological one. It's a bet on consistency. In a world of complex variables, PTFE is a constant. Its legendary non-reactivity isn't a feature; it's a promise of inaction, and in chemical handling, inaction is the ultimate virtue.
A Molecular Fortress by Design
The "magic" of PTFE isn't magic at all. It's the elegant, brute-force logic of its molecular structure—an engineer's romance written in chemistry.
A simple chain of carbon atoms is completely encased by a sheath of fluorine atoms. The carbon-fluorine bond is one of the strongest in organic chemistry. This creates a molecular shield, an impenetrable and non-reactive surface that is indifferent to almost any chemical assault.
Unwavering Chemical Inertness
This molecular shield renders PTFE practically immune to corrosion and degradation from the most aggressive acids, solvents, and bases. It doesn't yield, it doesn't rust, it doesn't care. It simply endures.
Resilience Across a Thermal Chasm
PTFE remains stable and functional across a vast temperature range, typically up to 200°C (392°F) in continuous service. This provides a crucial safety margin for processes involving exothermic reactions or high ambient temperatures, where lesser polymers would fail.
The Promise of Absolute Purity
The material is hydrophobic and does not absorb the substances it contains. Critically, it does not leach its own molecules into the fluid. For semiconductor or medical applications, where parts-per-billion contamination can be catastrophic, this non-contaminating property is non-negotiable.
From Theory to Reality: Where Trust is Built
These fundamental properties translate directly into the components that form the backbone of reliable chemical systems. Because of its unique strengths, PTFE is the default choice for the most vulnerable parts:
- Pipe Liners: Providing a non-reactive barrier inside a structurally rigid metal pipe.
- Seals & Gaskets: Ensuring leak-proof integrity at joints, the weakest points in any system.
- Valves & Pump Components: Protecting moving parts that are in constant contact with corrosive fluids.
- High-Purity Labware: Guaranteeing that experimental results are not tainted by the container itself.
The long, predictable service life of these components means less maintenance, less downtime, and a fundamentally safer operating environment.
An Honest Assessment: Understanding the Boundaries
No material is a panacea. The key to leveraging PTFE is to respect its limitations.
| Limitation | Design Consideration |
|---|---|
| Mechanical Softness | PTFE can be prone to "creep" (slow deformation) under sustained load. It's best used as a liner or in components where a stronger housing provides mechanical support. |
| High-Temp Decomposition | If heated far beyond its service limit (e.g., >400°C), it can decompose. Proper thermal management is essential. |
| Specialized Processing | It is a specialty polymer with a higher cost and cannot be melt-processed like common plastics, requiring expert fabrication techniques. |
The Final Step: From Raw Material to Reliable Component
The world's most inert polymer is only as reliable as the component it becomes. The transition from raw material to a mission-critical seal or a high-purity liner demands manufacturing expertise where tolerances are measured in microns and consistency is paramount.
A perfectly chosen material fabricated with the slightest imperfection is still a point of failure. This is why partnering with specialists is critical. KINTEK focuses exclusively on this transformation, fabricating precision PTFE components that uphold the material's promise of perfect integrity. We engineer and manufacture the seals, liners, and custom parts that allow engineers to trust their most critical systems.
Whether you are designing a system for ultra-pure laboratory reagents or aggressive industrial chemicals, the reliability of your components is paramount. Let our expertise in PTFE fabrication ensure your system's integrity from prototype to production. Contact Our Experts
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
Related Articles
- The PTFE Paradox: Why the 'Perfect' Material Fails—And How to Make It Work
- The Physics of a Perfect Fit: How PTFE Eliminates an Athlete's Hidden Distractions
- The Physics of Trust: Why PTFE Is the Bedrock of High-Stakes Electronics
- Beyond "Non-Stick": Why Your PTFE Components Fail and How to Fix It for Good
- How PTFE Solves Critical Industrial Challenges Through Material Superiority




















