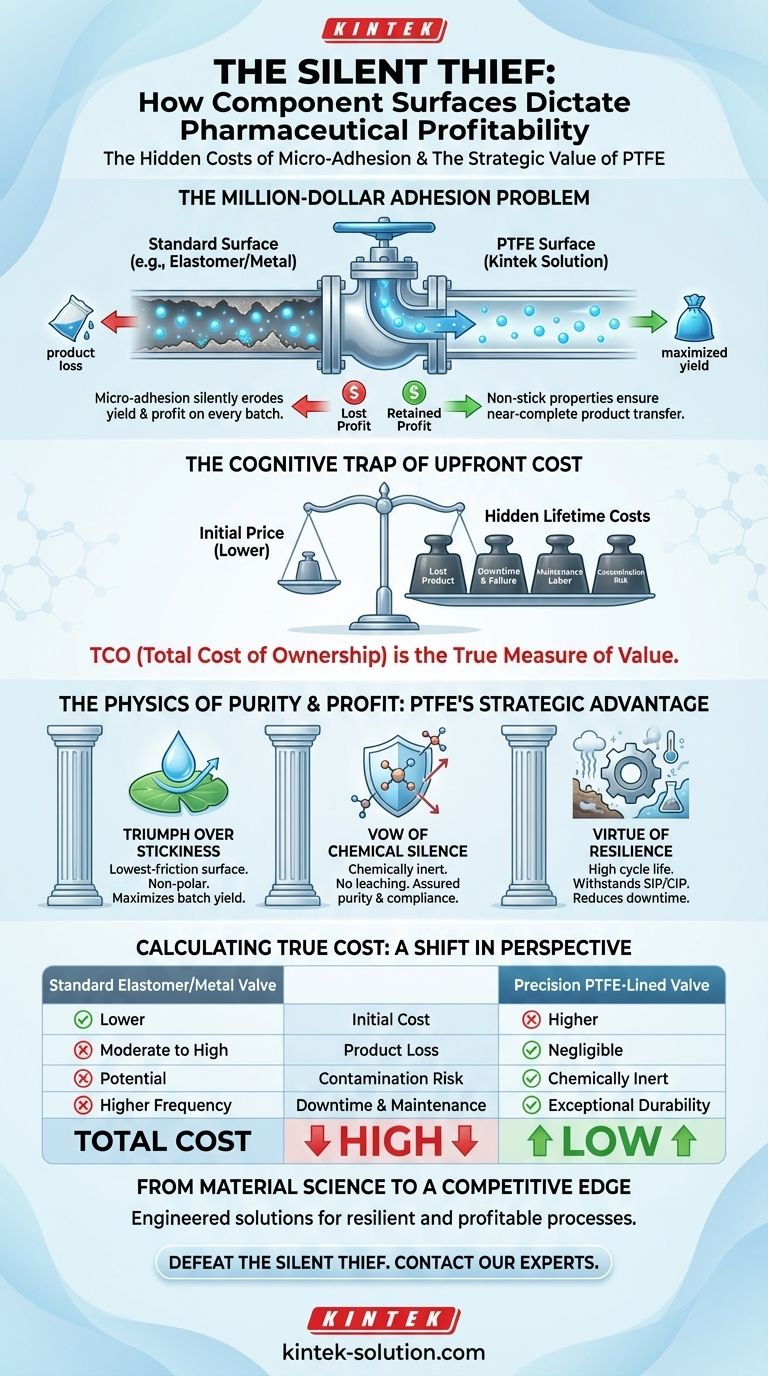
The Million-Dollar Adhesion Problem
Imagine a bioreactor holding a multi-million dollar batch of a life-saving biologic. The process is flawless, the parameters are perfect. Yet, at the end of the run, a small but significant percentage of the active pharmaceutical ingredient (API) is gone. It hasn't leaked. It wasn't destroyed. It simply stuck to the surfaces of the processing equipment.
This is the silent thief in pharmaceutical manufacturing: micro-adhesion. It's a tax levied by physics on every batch, silently eroding yields and profits. The culprit is often a component that was deemed "good enough"—a valve, a seal, a liner—chosen without a deep appreciation for the profound financial impact of its surface properties.
We tend to focus on catastrophic failures, but the more persistent threat is the slow, predictable drain caused by suboptimal material choices.
The Cognitive Trap of Upfront Cost
When specifying components like diaphragm valves, the human mind is naturally drawn to the most immediate and visible number: the purchase price. This creates a cognitive trap. A valve with a lower initial cost feels like a win for the budget, a responsible engineering decision.
But this perspective ignores the larger system. It fails to account for the hidden costs that accrue over the component's lifetime: the value of lost product that clings to its surface, the downtime from more frequent failures, the labor for constant maintenance, and the catastrophic risk of a contaminated batch.
The total cost of ownership (TCO) is a less intuitive, but far more accurate, measure of value. The most expensive component is often the one you have to replace, repair, or work around most often.
The Physics of Purity and Profit
The long-term business value of Polytetrafluoroethylene (PTFE) is not just a feature on a spec sheet; it's a direct consequence of its molecular structure. Understanding this connection reveals why it is a strategic asset in high-purity environments.
The Triumph Over Stickiness
Valuable APIs, especially in powder or viscous liquid form, are notoriously difficult to handle. They want to stick to surfaces. PTFE's surface is one of the lowest-friction surfaces known to science.
This isn't an accident. The fluorine atoms that sheath the polymer's carbon backbone create an incredibly low-energy, non-polar surface. Molecules have almost nothing to grab onto. This "non-stick" quality ensures a near-complete transfer of product through the valve, directly maximizing batch yield and minimizing waste.
The Vow of Chemical Silence
Purity is non-negotiable. The component material cannot react with the process media. PTFE is almost completely chemically inert because of the immense strength of its carbon-fluorine bonds.
It doesn't leach. It doesn't corrode. It doesn't interact. It acts as a silent, invisible conduit, ensuring that the only thing reaching the final product is the product itself. This property is a fundamental safeguard against contamination, protecting both patient safety and regulatory compliance.
The Virtue of Resilience
Pharmaceutical processes involve aggressive chemicals and harsh sterilization cycles (SIP/CIP). These conditions degrade lesser materials, leading to frequent failure, unplanned downtime, and production bottlenecks.
PTFE's durability and high cycle life mean its diaphragms and seals withstand these environments far longer. This reliability translates directly into more predictable operations, reduced maintenance schedules, and a lower risk of leakage-related safety incidents.
A Shift in Perspective: Calculating True Cost
Choosing a valve isn't just a component selection; it's an economic decision. The right framework moves beyond the initial price tag to evaluate the full operational impact.
| Feature | Standard Elastomer/Metal Valve | Precision PTFE-Lined Valve |
|---|---|---|
| Initial Cost | Lower | Higher |
| Product Loss | Moderate to High Adhesion | Negligible Adhesion (Higher Yield) |
| Contamination Risk | Potential for Leaching/Reaction | Chemically Inert (Assured Purity) |
| Downtime | Higher Frequency of Failure | Exceptional Durability & Cycle Life |
| Maintenance | Frequent Adjustments & Replacement | Minimal Interventions Required |
| Total Cost | High | Low |
While PTFE has its own operational limits, particularly for extreme temperature and pressure, applying it in the right context shifts a component from a recurring expense into a long-term strategic asset.
From Material Science to a Competitive Edge
The most elegant engineering solutions are often the simplest. In pharmaceutical manufacturing, the choice of a component's material is a decision that echoes through every part of the business—from production yield and product purity to operational efficiency and profitability.
At KINTEK, we don't just manufacture PTFE components; we engineer solutions based on a deep understanding of material science. Specializing in precision seals, liners, and custom-fabricated parts for the semiconductor, medical, and laboratory industries, we help our clients move beyond the spec sheet to build more resilient and profitable processes.
If you're ready to defeat the silent thief of inefficiency in your operations, let's talk about the physics of your process. Contact Our Experts
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
Related Articles
- The Unseen Guardian: How PTFE's Molecular Shield Protects Our Most Critical Systems
- Beyond "Non-Stick": Why Your PTFE Components Fail and How to Fix It for Good
- The PTFE Paradox: Why the 'Perfect' Material Fails—And How to Make It Work
- The Physics of a Perfect Fit: How PTFE Eliminates an Athlete's Hidden Distractions
- The Physics of Trust: Why PTFE Is the Bedrock of High-Stakes Electronics




















