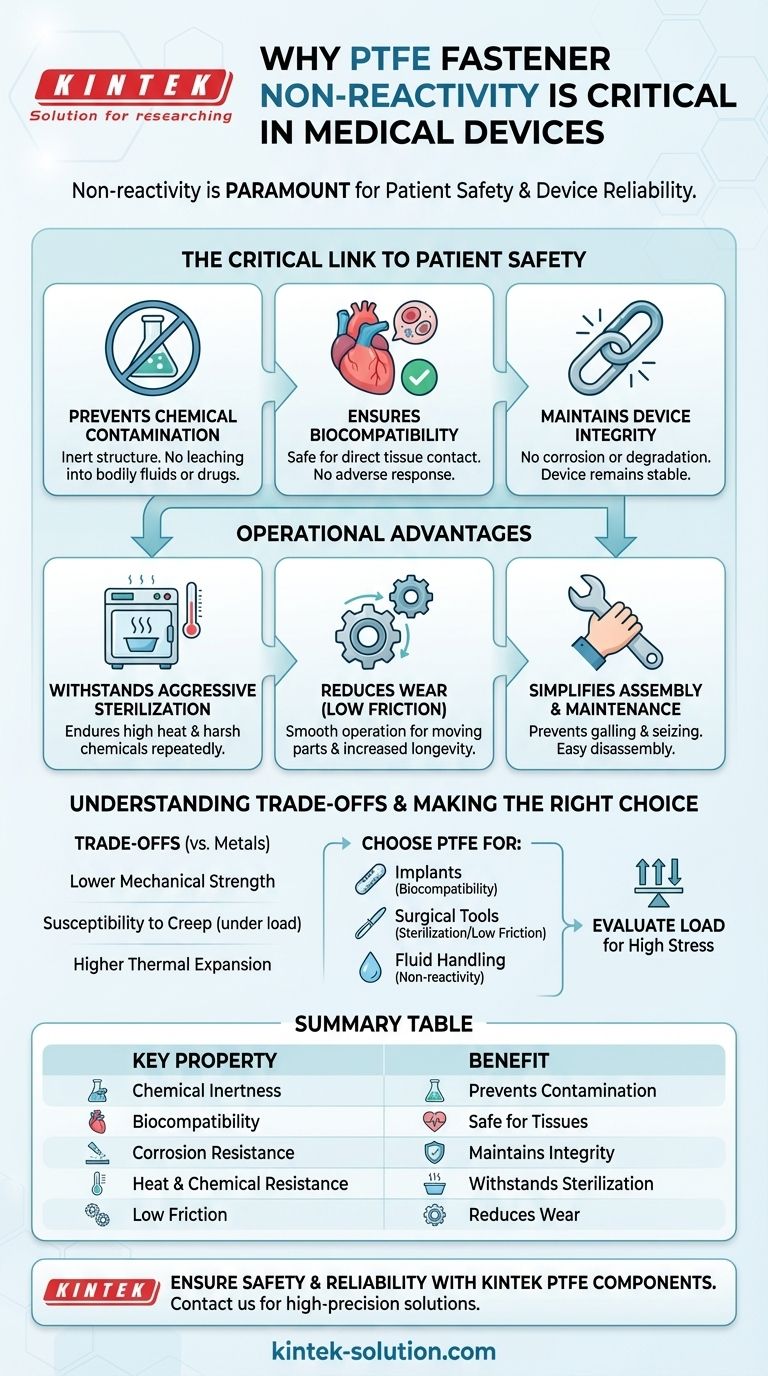
In medical device engineering, non-reactivity is paramount. The non-reactivity of PTFE fasteners is critical because it ensures the fastener will not chemically degrade or leach harmful substances when exposed to bodily fluids, aggressive sterilization agents, or pharmaceuticals. This core property directly prevents contamination, preserves the structural integrity of the device, and guarantees patient safety.
The non-reactivity of PTFE is not an isolated feature; it is the cornerstone of its suitability for medical applications. This chemical inertness is the foundation for its biocompatibility and corrosion resistance, ensuring a device remains stable, safe, and reliable throughout its lifecycle.

The Critical Link Between Non-Reactivity and Patient Safety
A material's interaction with its environment is a primary concern in medical technology. For fasteners, which hold critical components together, a lack of reaction is the most important reaction of all.
Preventing Chemical Contamination
PTFE's molecular structure makes it exceptionally stable and inert. It does not react with bodily fluids, diagnostic chemicals, or administered drugs.
This prevents the fastener from introducing any unintended or toxic substances into a patient's system or a sensitive laboratory sample, which is a fundamental requirement for safety and accuracy.
Ensuring Biocompatibility
Biocompatibility is essentially non-reactivity within a biological system. PTFE is widely recognized as safe for direct and prolonged contact with human tissues.
This property makes PTFE fasteners suitable for use in implants, surgical tools, and other devices where they may touch internal tissues without causing an adverse immune response.
Maintaining Device Integrity
A fastener that reacts to its environment will inevitably degrade. This corrosion or chemical breakdown compromises its mechanical properties.
Because PTFE does not corrode or degrade from chemical exposure, it maintains its strength and form. This ensures the medical device it is part of will not fail due to a weakened component.
Operational Advantages Beyond Chemical Inertness
While non-reactivity is the primary safety benefit, it enables other crucial operational properties that make PTFE fasteners highly effective in medical settings.
Withstanding Aggressive Sterilization
Medical devices must be sterilized, often using processes involving high heat, steam, and harsh chemicals.
PTFE's temperature resistance and chemical inertness mean it can endure these sterilization cycles repeatedly without degrading, ensuring the fastener remains reliable for the life of a reusable instrument.
Reducing Wear with Low Friction
PTFE has one of the lowest coefficients of friction of any solid material. This is vital for any device with moving parts, such as surgical instruments or diagnostic equipment.
This low-friction surface reduces wear and tear between components, leading to smoother operation and increased longevity for the entire device.
Simplifying Assembly and Maintenance
The low-friction nature of PTFE also prevents galling and seizing during assembly or disassembly.
This is a significant advantage for complex devices that require precise assembly or for instruments that must be taken apart frequently for cleaning and maintenance.
Understanding the Trade-offs
No material is perfect for every application. Objectively evaluating PTFE requires understanding its limitations compared to traditional metal fasteners like stainless steel or titanium.
Lower Mechanical Strength
As a polymer, PTFE has lower tensile and shear strength than metals. It is not suitable for applications that involve very high mechanical loads or stresses.
Engineers must carefully calculate the forces involved to ensure a PTFE fastener is appropriate for the specific joint it is securing.
Susceptibility to Creep
Under a constant, prolonged load, PTFE can slowly deform over time—a phenomenon known as "creep."
This must be considered in designs where consistent clamping pressure over many years is a critical requirement.
Higher Thermal Expansion
PTFE expands and contracts with temperature changes more than metals do.
For devices that experience significant thermal cycling, this difference in thermal expansion between the fastener and the surrounding materials must be accounted for in the design to prevent loosening or stress.
Making the Right Choice for Your Application
Choosing the appropriate fastener requires aligning its material properties with the specific demands of the device.
- If your primary focus is direct, long-term tissue contact (e.g., implants): PTFE's proven biocompatibility and chemical inertness are non-negotiable for ensuring patient safety.
- If your primary focus is reusable surgical instruments: The combination of chemical resistance to sterilization agents and a low-friction surface for moving parts makes PTFE an ideal choice.
- If your primary focus is fluid handling or diagnostic equipment: PTFE's non-reactivity is essential to prevent any contamination of sensitive samples, reagents, or therapeutic agents.
- If your primary focus is high mechanical load-bearing: You must evaluate whether PTFE's strength is sufficient, or if a metal fastener made of titanium or surgical steel is the more appropriate choice.
Ultimately, the non-reactive nature of PTFE is the core property that enables its other benefits, making it a foundational material for safe and reliable medical technology.
Summary Table:
| Key Property | Benefit in Medical Devices |
|---|---|
| Chemical Inertness | Prevents contamination from bodily fluids, drugs, and sterilants. |
| Biocompatibility | Safe for direct and prolonged contact with human tissues. |
| Corrosion Resistance | Maintains structural integrity and prevents device failure. |
| Heat & Chemical Resistance | Withstands repeated, aggressive sterilization cycles. |
| Low Friction | Reduces wear in moving parts and simplifies assembly. |
Ensure the Safety and Reliability of Your Medical Device
Choosing the right PTFE component is critical for patient safety and device performance. KINTEK specializes in manufacturing high-precision, medical-grade PTFE components—including fasteners, seals, and custom labware.
We prioritize precision production and offer custom fabrication from prototypes to high-volume orders, ensuring your components meet the stringent demands of the medical, semiconductor, and laboratory industries.
Contact us today to discuss your project requirements and receive a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
People Also Ask
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability



















