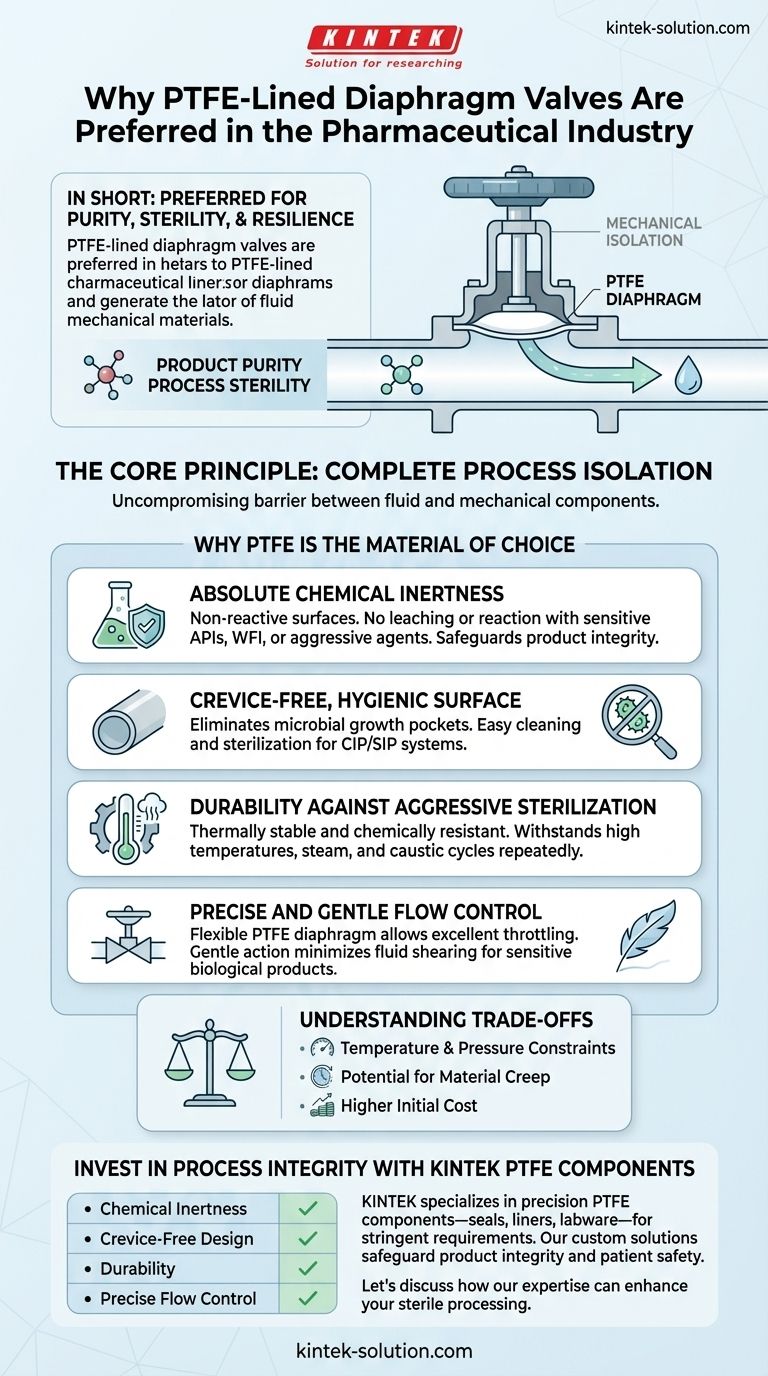
In short, PTFE-lined diaphragm valves are preferred in the pharmaceutical industry for their unparalleled ability to ensure product purity and maintain process sterility. Their non-reactive surfaces, crevice-free hygienic design, and resilience to aggressive cleaning cycles make them the definitive choice for handling high-purity and sensitive substances without risk of contamination.
The decision to use a PTFE-lined diaphragm valve is fundamentally a strategy to mitigate risk. Its material properties and mechanical design directly address the pharmaceutical industry's most critical demands: safeguarding product integrity, ensuring patient safety, and upholding operational efficiency in sterile environments.

The Core Principle: Complete Process Isolation
To understand the preference for these valves, you first have to understand their fundamental design. They are engineered to create an uncompromising barrier between the fluid being processed and the valve's mechanical components.
How a Diaphragm Valve Works
A diaphragm valve uses a flexible, engineered membrane (the diaphragm) to control flow. This diaphragm is pressed down to create a seal against the valve body, stopping flow, and lifted to allow it.
Critically, this design means the substance being processed only ever touches the valve body and the diaphragm itself. The valve stem, actuator, and other moving parts are completely isolated from the fluid path.
The Critical Role of the PTFE Lining
The diaphragm is the heart of the valve, and PTFE (Polytetrafluoroethylene) is the material of choice for lining it in pharmaceutical applications.
PTFE provides a combination of properties that are perfectly suited to the industry's stringent requirements, transforming a standard valve into a component capable of maintaining absolute purity.
Why PTFE Is the Material of Choice for Pharma
The specific characteristics of PTFE directly solve the most significant challenges in pharmaceutical manufacturing, from handling costly Active Pharmaceutical Ingredients (APIs) to ensuring a sterile final product.
Absolute Chemical Inertness
Pharmaceutical processes involve high-purity water (WFI), sensitive biological materials, and aggressive chemical agents.
PTFE is one of the most non-reactive materials known. It will not leach substances into the product or react with the media, which is a non-negotiable requirement for preventing contamination and preserving product integrity.
A Crevice-Free, Hygienic Surface
The smooth, non-porous surface of PTFE is highly resistant to adhesion. This "no-pocket" or crevice-free quality is vital for two reasons.
First, it prevents microbial growth by eliminating areas where bacteria could colonize. Second, it makes the valve exceptionally easy to clean and sterilize during Clean-in-Place (CIP) and Sterilize-in-Place (SIP) procedures.
Durability Against Aggressive Sterilization
Pharmaceutical equipment must be cleaned and sterilized frequently and aggressively. This involves high temperatures, steam, and caustic cleaning solutions.
PTFE is thermally stable and chemically resistant, allowing it to withstand these harsh cycles repeatedly without degrading. This ensures a long service life and reduces costly maintenance and downtime.
Precise and Gentle Flow Control
The flexible nature of the PTFE-lined diaphragm allows for excellent throttling capabilities. This provides the precise flow control needed for sensitive processes.
Furthermore, the gentle opening and closing action minimizes fluid shearing, which is crucial when handling delicate cell cultures or other sensitive biological products.
Understanding the Trade-offs
While highly effective, PTFE-lined diaphragm valves are not without their limitations. Objectivity requires acknowledging their specific operational boundaries.
Temperature and Pressure Constraints
Compared to all-metal valves, PTFE has a more limited operating temperature and pressure range. Exceeding these specifications can compromise the integrity of the diaphragm and the seal.
Potential for Material Creep
PTFE is a relatively soft material. Under sustained high pressure or stress, it can be subject to "creep," or slow deformation over time. This can eventually affect the valve's sealing performance, necessitating periodic inspection and replacement.
Higher Initial Cost
The specialized materials and hygienic design of these valves often result in a higher upfront cost compared to simpler industrial valves. However, this is typically offset by lower long-term costs due to reduced batch contamination risk, less maintenance, and greater reliability.
Making the Right Choice for Your Application
Selecting the correct valve is about aligning its capabilities with your primary process goal.
- If your primary focus is sterility and cleanability: The crevice-free, non-porous surface of a PTFE diaphragm is the superior choice for preventing contamination in CIP/SIP systems.
- If your primary focus is handling aggressive chemicals or APIs: The absolute chemical inertness of PTFE ensures there is no reaction, leaching, or degradation of the product or the valve.
- If your primary focus is long-term reliability and process uptime: The high cycle life and durability of these valves against harsh cleaning cycles minimize maintenance and reduce the risk of costly equipment failure.
Ultimately, selecting a PTFE-lined diaphragm valve is a deliberate investment in process integrity, operational stability, and patient safety.
Summary Table:
| Key Advantage | Benefit for Pharma |
|---|---|
| Chemical Inertness | Prevents contamination; safe for sensitive APIs and WFI. |
| Crevice-Free Design | Eliminates microbial growth; ideal for CIP/SIP systems. |
| Durability | Withstands aggressive sterilization cycles, reducing downtime. |
| Precise Flow Control | Gentle handling for delicate biological products. |
Invest in Process Integrity with KINTEK PTFE Components
Your pharmaceutical processes demand the highest level of purity and reliability. KINTEK specializes in manufacturing precision PTFE components—including seals, liners, and custom labware—specifically for the stringent requirements of the semiconductor, medical, and laboratory industries.
We understand that your primary focus is on sterility, cleanability, and handling sensitive substances. Our custom fabrication services, from prototypes to high-volume orders, ensure you get components that perfectly align with your application's critical needs, safeguarding product integrity and patient safety.
Let's discuss how our PTFE expertise can enhance your sterile processing.
Contact KINTEK today for a consultation
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE and Nitrile Diaphragm Pump Components for Demanding Applications
People Also Ask
- What challenges arise when machining PTFE (Teflon)? Overcome Softness, Heat, and Instability
- What finishing techniques are effective for machined Teflon parts? Achieve Functional Performance and Dimensional Stability
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application
- What are the unique properties of PTFE? The 3 Pillars Driving Demand for High-Performance Parts
- What chemical processing applications involve PTFE-machined parts? Essential Components for Corrosive & High-Purity Systems



















