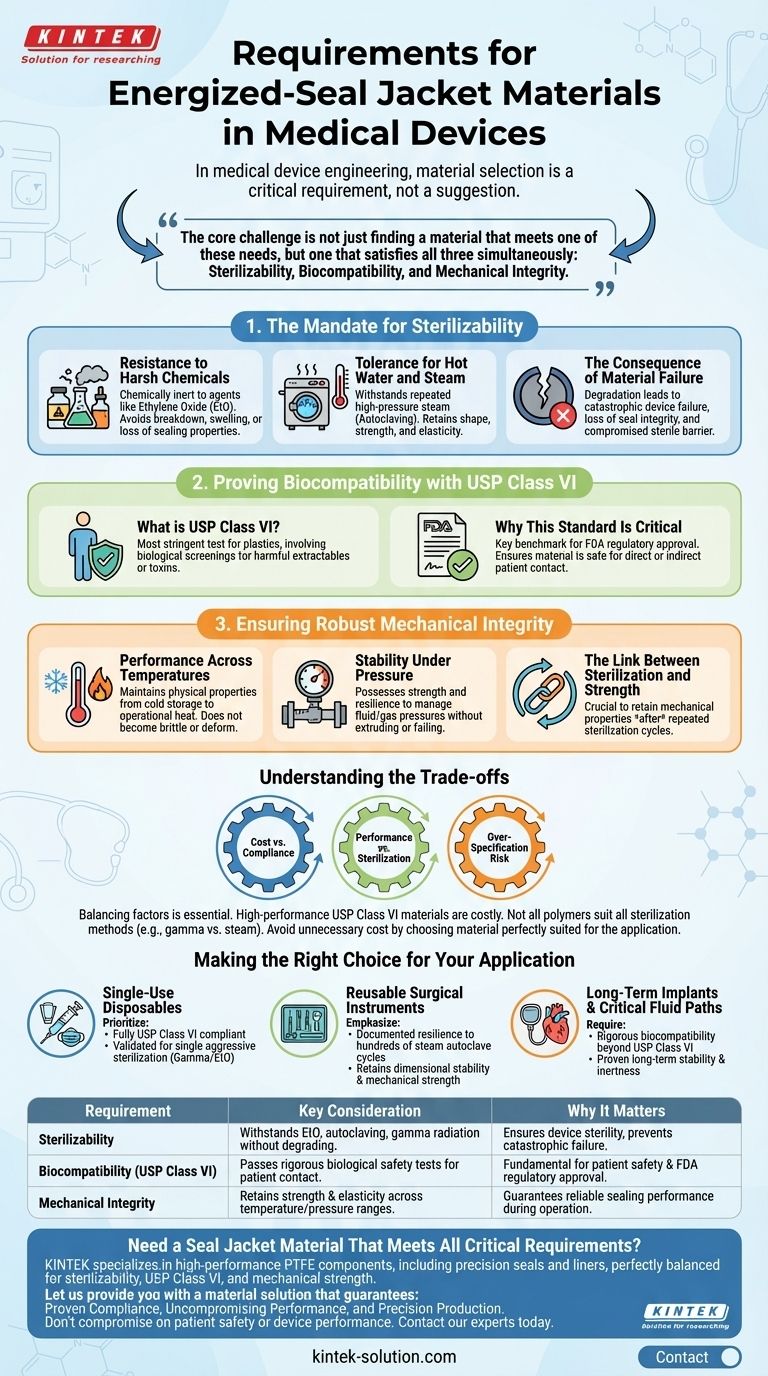
In medical device engineering, material selection is not a suggestion—it is a critical requirement. An energized-seal jacket material must meet three fundamental criteria: it must be fully sterilizable, it must qualify as a USP Class VI material for biocompatibility, and it must retain its mechanical strength across a wide range of operating temperatures and pressures.
The core challenge is not just finding a material that meets one of these needs, but one that satisfies all three simultaneously. A material's strength is irrelevant if it cannot be sterilized, and its ability to be sterilized is useless if it is not safe for patient contact.

The Mandate for Sterilizability
Sterilization is a non-negotiable process intended to eliminate all microorganisms. The seal jacket material must endure these aggressive procedures without degrading, as any failure could compromise the sterility and function of the entire device.
Resistance to Harsh Chemicals
Many medical devices are sterilized using potent chemical agents, such as ethylene oxide (EtO). The seal material must be chemically inert to these substances to avoid breaking down, swelling, or losing its sealing properties.
Tolerance for Hot Water and Steam
Autoclaving, which uses high-pressure steam, is one of the most common and effective sterilization methods. Seal jackets must withstand repeated cycles of high temperatures and moisture without losing their shape, strength, or elasticity.
The Consequence of Material Failure
If a seal's material degrades during sterilization, it can lead to catastrophic device failure. This could manifest as a loss of seal integrity, the release of harmful particulates, or a compromised sterile barrier.
Proving Biocompatibility with USP Class VI
Biocompatibility ensures that a material will not cause an adverse reaction when it comes into contact with the human body. For medical devices, this is a fundamental aspect of patient safety.
What is USP Class VI?
The United States Pharmacopeia (USP) Class VI classification is the most stringent test for plastics. It involves a series of biological screenings to determine if a material releases any harmful extractables or toxins when exposed to bodily fluids.
Why This Standard Is Critical
Meeting the USP Class VI standard is a key benchmark for regulatory bodies like the FDA. It provides a high degree of confidence that the material is safe for use in medical applications, including those involving direct or indirect patient contact.
Ensuring Robust Mechanical Integrity
A seal's primary function is to prevent leaks under operational stress. This requires the jacket material to maintain its physical properties in the demanding environment of a medical device.
Performance Across Temperatures
Medical devices can be exposed to a wide thermal range, from cold storage to the heat of operation or sterilization. The material must not become brittle at low temperatures or soften and deform at high temperatures.
Stability Under Pressure
Seals are often used in systems that manage fluids or gases, subjecting them to constant or fluctuating pressures. The jacket material must possess the strength and resilience to maintain a consistent seal without extruding or failing.
The Link Between Sterilization and Strength
Crucially, the material must retain its mechanical properties after undergoing sterilization. The true test of a material is not its strength off the shelf, but its strength after being subjected to the harsh chemical or thermal stresses of repeated sterilization cycles.
Understanding the Trade-offs
Selecting the right material involves balancing competing factors. An ideal choice for one requirement may present a challenge for another, making a holistic view essential.
Cost vs. Regulatory Compliance
Materials that meet USP Class VI and offer exceptional thermal and chemical resistance are inherently more expensive. Attempting to reduce costs with a non-compliant material introduces unacceptable risks to patient safety and guarantees regulatory rejection.
Performance vs. Sterilization Method
Not all high-performance polymers are compatible with all sterilization methods. For example, a material with excellent mechanical properties may degrade under gamma radiation but perform perfectly in a steam autoclave. The material and the sterilization process must be chosen in tandem.
The Risk of Over-Specification
While safety is paramount, choosing a material that far exceeds the application's requirements can add unnecessary cost and manufacturing complexity. The goal is to select a material that is perfectly suited and proven for its specific intended use.
Making the Right Choice for Your Application
Your final material decision should be guided by the specific demands of your medical device and its lifecycle.
- If your primary focus is single-use disposable devices: Prioritize materials that are fully compliant with USP Class VI and validated for a single, aggressive sterilization method like gamma or EtO.
- If your primary focus is reusable surgical instruments: Emphasize materials with documented resilience to hundreds of steam autoclave cycles without losing dimensional stability or mechanical strength.
- If your primary focus is long-term implants or critical fluid paths: Require rigorous biocompatibility data that goes beyond USP Class VI and proves the material's long-term stability and inertness.
Ultimately, choosing the right seal jacket material is a foundational engineering decision that directly impacts patient safety, device reliability, and regulatory success.
Summary Table:
| Requirement | Key Consideration | Why It Matters |
|---|---|---|
| Sterilizability | Must withstand EtO, autoclaving, or gamma radiation without degrading. | Ensures device sterility and prevents catastrophic failure. |
| Biocompatibility (USP Class VI) | Passes rigorous biological safety tests for patient contact. | Fundamental for patient safety and FDA regulatory approval. |
| Mechanical Integrity | Retains strength and elasticity across a wide temperature and pressure range. | Guarantees reliable sealing performance during device operation. |
Need a Seal Jacket Material That Meets All Critical Requirements?
Selecting the right material is a foundational decision for your medical device's safety, reliability, and regulatory success. KINTEK specializes in manufacturing high-performance PTFE components, including precision seals and liners, for the most demanding applications.
We understand the critical balance between sterilizability, USP Class VI biocompatibility, and mechanical strength. Our expertise ensures your components are perfectly suited for their intended use, from single-use disposables to reusable surgical instruments.
Let us provide you with a material solution that guarantees:
- Proven Compliance: Materials validated for critical standards.
- Uncompromising Performance: Components that maintain integrity after repeated sterilization cycles.
- Precision Production: Custom fabrication from prototypes to high-volume orders.
Don't compromise on patient safety or device performance. Contact our experts today to discuss your specific application requirements.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Customizable PTFE Rods for Advanced Industrial Applications
People Also Ask
- What are the primary applications of PTFE fasteners and custom parts? Critical Solutions for Extreme Environments
- What are the key benefits of PTFE in custom fabrication? Unlock Performance in Extreme Conditions
- What are some common applications of machined PTFE? Leverage its Unique Properties for Demanding Applications
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications
- What is PTFE commonly known as and what are its unique properties? Unlock Unmatched Chemical & Thermal Resistance



















