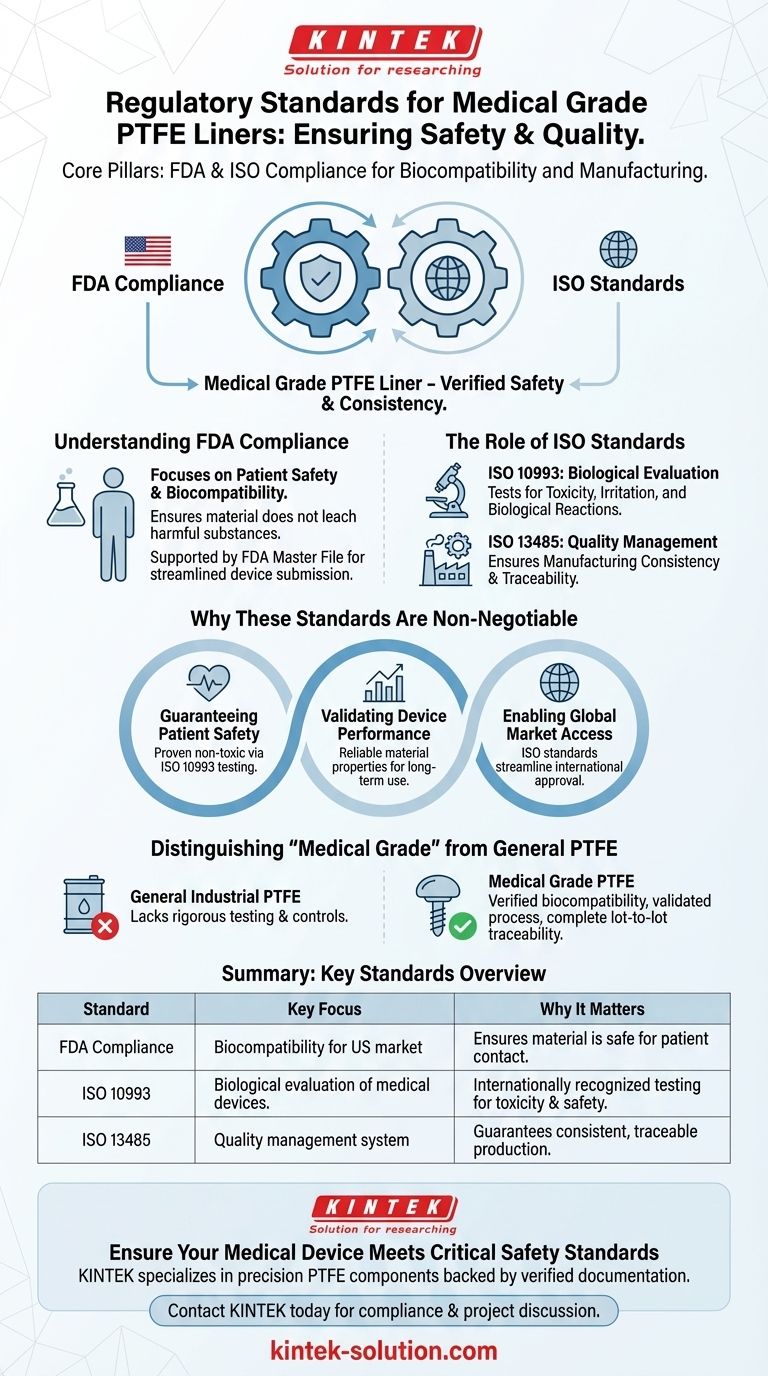
At its core, Medical Grade Polytetrafluoroethylene (PTFE) Liners must meet rigorous standards set by two primary bodies: the U.S. Food and Drug Administration (FDA) and the International Organization for Standardization (ISO). These certifications are not optional; they are the fundamental requirements that ensure the material is safe, reliable, and suitable for critical medical device applications. Compliance confirms the material's biocompatibility and the consistency of its manufacturing process.
The crucial takeaway is that "Medical Grade" is not just a marketing term. It signifies a verified level of safety and quality, primarily defined by compliance with ISO 10993 for biocompatibility and adherence to FDA regulations for materials intended for medical use.

The Core Regulatory Pillars: FDA and ISO
To understand the value of a medical-grade liner, you must understand the protections these two standards provide. They work in tandem to guarantee both material safety and manufacturing quality.
Understanding FDA Compliance
The FDA regulates materials used in medical devices to ensure patient safety. For PTFE liners, this means the material is considered biocompatible and does not leach harmful substances.
FDA compliance is often supported by a Master File, which allows a manufacturer to submit confidential data about their material directly to the agency for review in support of a customer's device submission.
The Role of ISO Standards
ISO standards provide an international framework for quality and safety. The most critical standard for medical materials like PTFE is ISO 10993, "Biological evaluation of medical devices."
This standard outlines a series of tests to confirm a material will not cause any adverse biological reactions, such as toxicity, irritation, or other harm when it comes into contact with the human body.
Ensuring Manufacturing Consistency
Beyond the material itself, standards like ISO 13485 specify the requirements for a quality management system for medical device manufacturing.
A supplier compliant with this standard demonstrates they have robust processes in place to ensure every liner is produced with the same quality, purity, and traceability as the one that was tested and approved.
Why These Standards Are Non-Negotiable
Compliance is the bedrock of medical device development. It directly impacts patient outcomes, device performance, and your ability to bring a product to market.
Guaranteeing Patient Safety
The primary function of these regulations is to protect patients. Biocompatibility testing according to ISO 10993 is the most direct way to prove that a PTFE liner is non-toxic and safe for its intended application.
Validating Device Performance and Longevity
The inherent properties of PTFE—such as its durability, chemical inertness, and low water absorption—make it ideal for long-term medical use.
Regulatory compliance ensures these material properties are consistently present and reliable from batch to batch, guaranteeing the device functions as designed throughout its entire lifecycle.
Enabling Global Market Access
While the FDA governs the U.S. market, ISO is a globally recognized standard. Adherence to ISO 10993 and ISO 13485 streamlines the regulatory approval process in numerous countries across Europe, Asia, and beyond.
Distinguishing "Medical Grade" from General PTFE
A common pitfall is assuming any PTFE is suitable for a medical application. The distinction between general-purpose and medical-grade PTFE is critical and has significant regulatory implications.
It’s About Control, Not Just Chemistry
Standard industrial PTFE might meet some general specifications (e.g., for food contact), but it lacks the rigorous testing and manufacturing controls required for medical use.
"Medical Grade" signifies that the material has passed specific biocompatibility tests and was manufactured under a validated, controlled, and traceable process.
The Critical Importance of Traceability
Medical-grade components require complete lot-to-lot traceability, from the raw polymer resin to the final, packaged liner.
This ensures that if an issue ever arises, the affected batch can be quickly and precisely identified, which is a core requirement of any medical quality system.
What to Demand from a Supplier
Always request documentation that explicitly states compliance with ISO 10993. Do not accept vague claims of "meets industry standards."
Furthermore, verify that your supplier operates under a certified quality management system, such as ISO 13485, to ensure process control and consistency.
How to Verify Compliance for Your Application
Choosing the right material requires asking for the right proof. Your specific goal will determine which documentation is most critical.
- If your primary focus is patient safety and biocompatibility: Demand a certificate of compliance that specifically references ISO 10993 testing for your liner.
- If your primary focus is consistent manufacturing and quality control: Ensure your supplier is certified to ISO 13485 or an equivalent medical device quality management system.
- If your primary focus is US market approval: Confirm the material is supported by an FDA Master File to simplify your device submission process.
Ultimately, selecting a liner with verified compliance is a foundational step in mitigating risk and ensuring the success of your medical device.
Summary Table:
| Standard | Key Focus | Why It Matters |
|---|---|---|
| FDA Compliance | Biocompatibility for US market | Ensures material is safe for patient contact and doesn't leach harmful substances. |
| ISO 10993 | Biological evaluation of medical devices | Internationally recognized testing for toxicity, irritation, and safety. |
| ISO 13485 | Quality management system for manufacturing | Guarantees consistent, traceable, and high-quality production of every liner. |
Ensure Your Medical Device Meets Critical Safety Standards
Navigating FDA and ISO compliance for your PTFE components is complex, but you don't have to do it alone. KINTEK specializes in manufacturing precision PTFE seals, liners, and labware that meet the rigorous demands of the medical, semiconductor, and laboratory industries.
We provide the verified documentation and material traceability you need to streamline your device approval process. From prototypes to high-volume orders, our custom fabrication is backed by a commitment to quality and safety.
Let us be your trusted partner in compliance. Contact KINTEB today to discuss your project requirements and ensure your components are built to the highest standards.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the unique properties of PTFE? Unlock Unmatched Performance in Demanding Applications
- What are the material advantages of machining Teflon? Unlock Unmatched Chemical & Thermal Resistance
- Why is PTFE suitable for cryogenic or high-temperature applications? Unmatched Thermal Stability from -450°F to 500°F
- What is the working temperature range of PTFE? Master Extreme Heat and Cryogenic Applications



















