At its core, the suitability of PTFE envelope gaskets for food and pharmaceutical applications stems from a unique combination of material properties. They are fundamentally non-toxic, chemically inert, and FDA-compliant for direct contact with consumables. These characteristics ensure that the gasket itself does not become a source of contamination, which is the paramount concern in these highly regulated industries.
The critical insight is that PTFE's value extends beyond mere regulatory compliance. Its inherent properties—chemical inertness, temperature resistance, and a non-stick surface—directly solve the core challenges of sanitary processing: guaranteeing product purity, enabling effective sterilization, and maintaining operational efficiency.

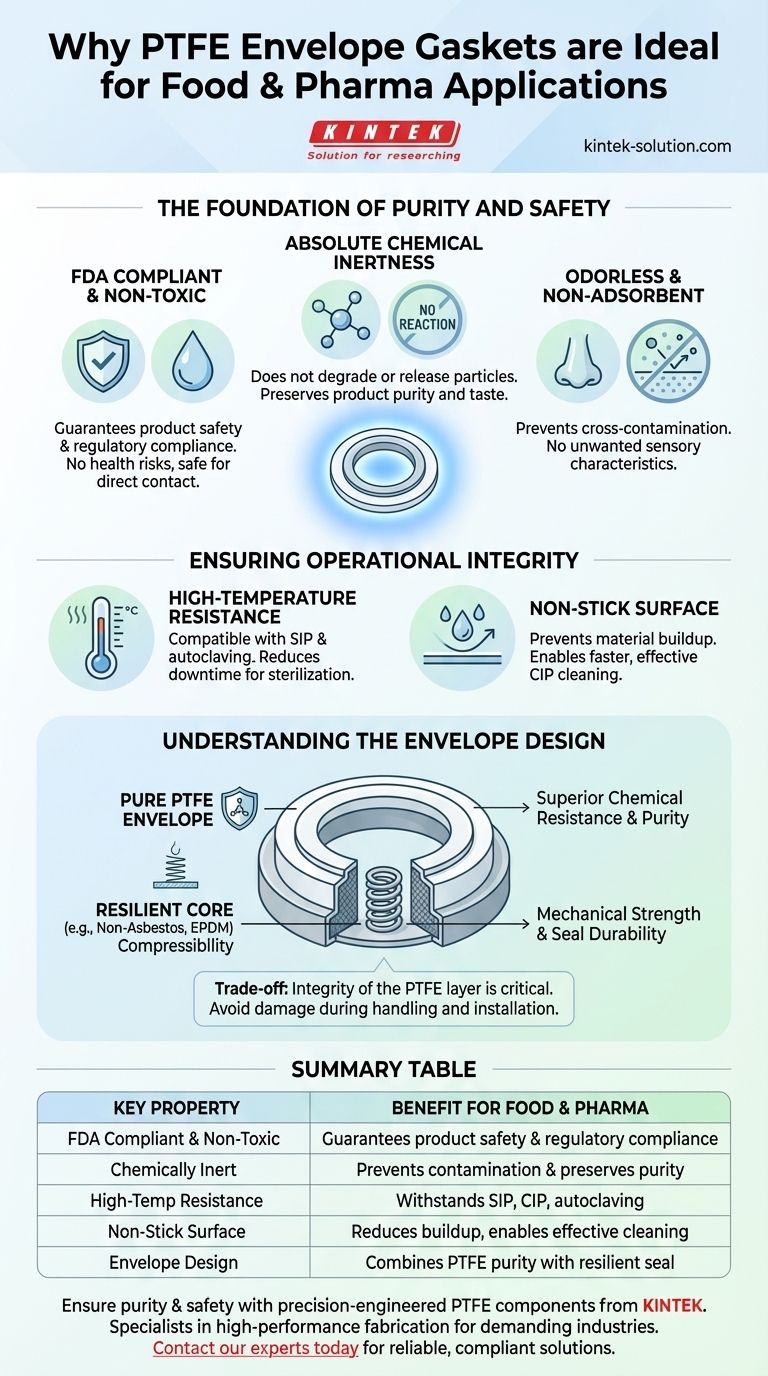
The Foundation of Purity and Safety
In food and pharmaceutical manufacturing, any material that touches the product must not compromise its integrity. PTFE's properties provide this fundamental guarantee.
FDA Compliance and Non-Toxicity
The U.S. Food and Drug Administration (FDA) recognizes PTFE as a material safe for direct contact with food and drugs. This compliance is the baseline requirement for any component used in sanitary processing equipment.
Because PTFE is non-toxic and does not contain substances that can leach out, it poses no health risk when used in machinery for processing, manufacturing, or packaging consumables.
Absolute Chemical Inertness
PTFE is one of the most chemically inert polymers known. It does not react with the vast majority of chemicals, ingredients, or active pharmaceutical ingredients (APIs).
This non-reactive nature ensures the gasket will not degrade, break down, or release particles into the product stream. This preserves the purity, taste, and chemical composition of the final product.
Odorless and Non-Adsorbent
The material is also odorless and tasteless, meaning it will not impart any unwanted sensory characteristics to the product.
Furthermore, PTFE is non-adsorbent. It does not trap or retain particles from the media flowing past it, which is critical for preventing cross-contamination between different product batches.
Ensuring Operational Integrity
Beyond safety, PTFE gaskets contribute directly to the efficiency and reliability of manufacturing operations, particularly regarding cleaning and sterilization.
High-Temperature Resistance for Sterilization
PTFE can withstand a wide range of temperatures, making it fully compatible with standard sterilization procedures like Steam-in-Place (SIP) and autoclaving.
This thermal stability allows equipment to be thoroughly sterilized without needing to remove the gaskets, significantly reducing downtime and ensuring a consistently hygienic environment.
The Advantage of a Non-Stick Surface
PTFE is famous for its non-stick (low friction) surface. In a processing line, this property prevents materials from building up on the gasket surface.
This not only prevents a potential source of contamination but also makes cleaning processes, such as Clean-in-Place (CIP), faster and more effective.
Understanding the Trade-offs: The Envelope Design
While PTFE offers superior chemical and purity benefits, it is a relatively soft material. The "envelope" design is an intelligent engineering compromise that addresses this.
Why an "Envelope"?
Pure PTFE gaskets can be susceptible to cold flow or "creep," where the material slowly deforms under pressure, potentially leading to a loss of seal.
An envelope gasket solves this by using a mechanically robust core material (such as compressed non-asbestos fiber or EPDM rubber) to provide compressibility and resilience. This core is then fully encapsulated in a thin "envelope" of pure PTFE.
This design delivers the superior chemical resistance and purity of a PTFE-wetted surface while relying on the inner core material for the mechanical strength needed to create and maintain a durable seal.
Potential Weaknesses
The primary trade-off is the integrity of the envelope itself. If the PTFE layer is scratched, punctured, or improperly installed, the core material can be exposed to the process media.
This exposure would negate the benefits of the PTFE and could introduce a source of contamination. Therefore, careful handling and correct installation torque are critical to the gasket's performance and longevity.
Making the Right Choice for Your Application
Selecting the correct gasket requires balancing the demands of product purity with mechanical sealing requirements.
- If your primary focus is product purity and regulatory compliance: The PTFE surface is non-negotiable, as its inertness and FDA-compliant status are unmatched.
- If your primary focus is operational efficiency: The high-temperature and non-stick properties of PTFE are key for minimizing downtime associated with SIP/CIP cycles.
- If your primary focus is robust mechanical sealing under high pressure or temperature cycling: Pay close attention to the core material of the envelope gasket to ensure it provides the necessary resilience and recovery.
By understanding how the envelope design combines material strengths, you can confidently select a gasket that ensures both safety and operational reliability.
Summary Table:
| Key Property | Benefit for Food & Pharma Applications |
|---|---|
| FDA Compliant & Non-Toxic | Guarantees product safety and regulatory compliance |
| Chemically Inert | Prevents contamination and preserves product purity |
| High-Temperature Resistance | Withstands SIP, CIP, and autoclaving sterilization |
| Non-Stick Surface | Reduces buildup and enables effective cleaning |
| Envelope Design | Combines PTFE purity with a resilient core for a durable seal |
Ensure the purity and safety of your food or pharmaceutical processes with precision-engineered PTFE components from KINTEK.
As specialists in high-performance PTFE fabrication, we manufacture seals, liners, and labware that meet the stringent demands of the semiconductor, medical, and laboratory industries. Our custom fabrication services, from prototypes to high-volume orders, are designed to provide you with reliable, compliant solutions that protect your product integrity.
Contact our experts today to discuss your specific application requirements and receive a custom solution that guarantees performance and compliance.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What is PTFE commonly known as and what are its unique properties? Unlock Unmatched Chemical & Thermal Resistance
- What are some common applications of machined PTFE? Leverage its Unique Properties for Demanding Applications
- What are the key benefits of PTFE in custom fabrication? Unlock Performance in Extreme Conditions
- What is the working temperature range of PTFE? Master Extreme Heat and Cryogenic Applications
- What are the base characteristics of PTFE? Unlocking Extreme Performance in Friction, Temperature, and Chemical Resistance



















