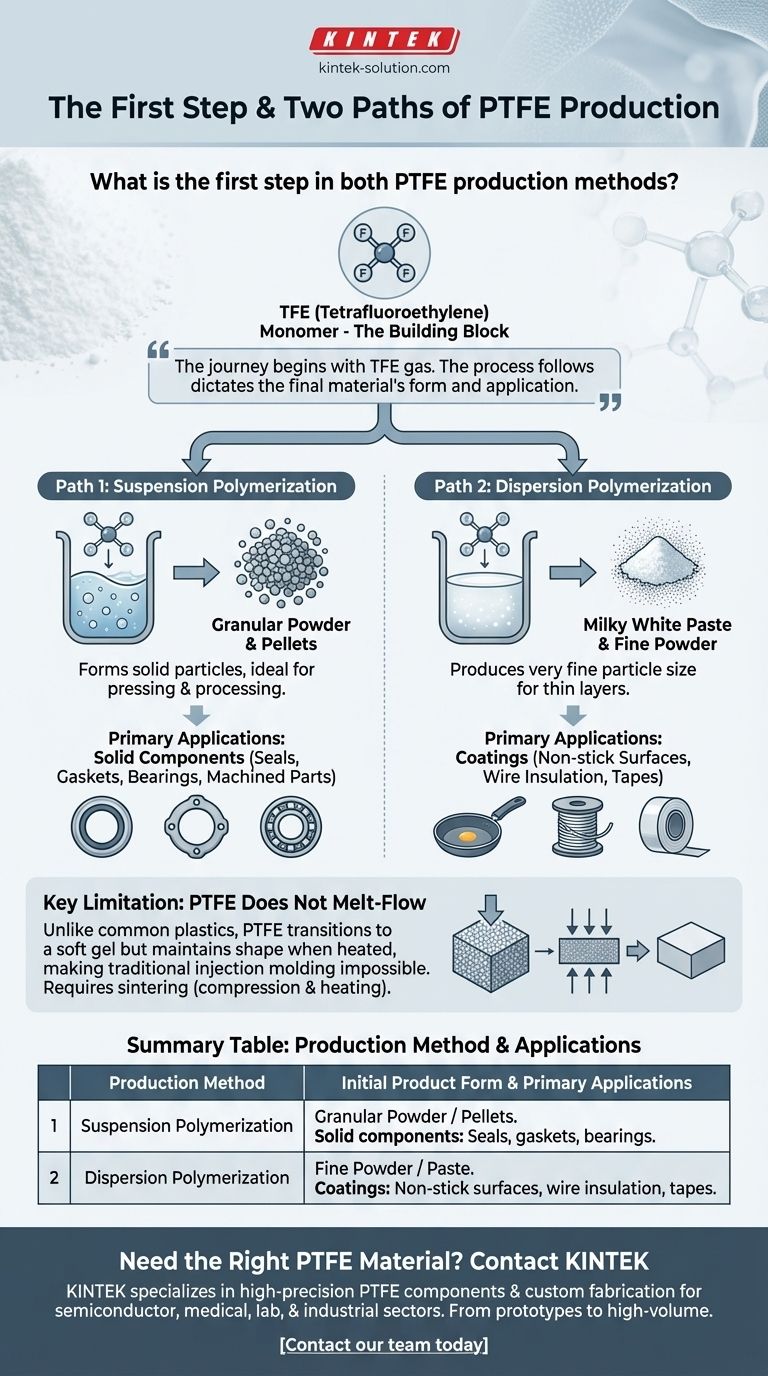
The first step in both common Polytetrafluoroethylene (PTFE) production methods is the creation of its core chemical building block: TFE (tetrafluoroethylene). This colorless, odorless gas is the monomer from which the long polymer chains of PTFE are constructed.
The journey to create any PTFE product, whether a solid machined part or a non-stick coating, begins with the same starting block—a gas called TFE. The specific manufacturing process that follows, either suspension or dispersion polymerization, fundamentally dictates the final material's physical form and its ultimate application.

The Two Paths of PTFE Production
All PTFE is created by linking individual TFE gas molecules (monomers) into long, stable chains (a polymer). This process is called polymerization. The specific environment in which this linking occurs determines the final product's characteristics.
Path 1: Suspension Polymerization
Suspension polymerization involves polymerizing the TFE monomer in water. This process creates solid particles of PTFE.
The resulting material is a granular powder or grain. These grains can be pressed and processed into solid pellets, which are ideal for further manufacturing.
Path 2: Dispersion Polymerization
The dispersion method also uses water but produces a much finer particle size. This results in a completely different initial form.
The output is a milky white paste or a fine powder. This form is not intended for creating large, solid blocks but is perfect for creating thin layers and coatings.
Why the Production Method Matters
The choice between suspension and dispersion is not arbitrary; it's driven entirely by the intended end-use of the PTFE. The initial form dictates how the material can be processed and what it can become.
Granular PTFE for Solid Components
PTFE produced via suspension polymerization is destined to become solid objects. The resulting pellets are shaped through methods like molding and machining.
This is the path used to create robust parts like seals, gaskets, bearings, and electrical insulators.
Fine Powder & Paste for Coatings
PTFE produced via dispersion polymerization is used for surface applications. The paste can be spread or sprayed to create the non-stick coatings found on cookware.
The fine powder can also be used for applications like wire insulation or creating tapes.
Understanding the Key Limitation
A critical characteristic of PTFE governs its entire manufacturing landscape and explains why these two distinct methods are necessary.
PTFE Does Not Melt-Flow
Unlike common plastics that can be melted and injected into a mold, PTFE does not flow when it melts. It transitions into a soft gel but maintains its shape.
This behavior makes traditional injection molding impossible. Consequently, creating solid parts requires compressing the granular powder and then heating it in a process called sintering.
Customization Through Processing
Because of these unique properties, the final mechanical and physical characteristics of a PTFE part can be heavily influenced by the specific molding and sintering methods used. This allows for a degree of customization based on the application's demands.
Making the Right Choice for Your Goal
The initial production method is a direct reflection of the final goal.
- If your primary focus is creating solid, structural components: You need granular PTFE derived from suspension polymerization, which is suitable for molding and machining.
- If your primary focus is applying a thin, non-stick layer or coating: You need the fine powder or paste derived from dispersion polymerization.
Understanding this fundamental split in production is the key to sourcing the correct material for any engineering application.
Summary Table:
| Production Method | Initial Product Form | Primary Applications |
|---|---|---|
| Suspension Polymerization | Granular Powder / Pellets | Solid components: Seals, gaskets, bearings, machined parts |
| Dispersion Polymerization | Fine Powder / Paste | Coatings: Non-stick surfaces, wire insulation, tapes |
Need the Right PTFE Material for Your Application?
Understanding the production method is the first step to selecting the perfect PTFE for your project. At KINTEK, we specialize in manufacturing high-precision PTFE components for the semiconductor, medical, laboratory, and industrial sectors.
Whether you require custom-machined seals and liners from granular PTFE or specialized coatings from dispersion PTFE, our expertise in custom fabrication—from prototypes to high-volume orders—ensures you get a component that meets your exact specifications.
Let's discuss your project requirements. Contact our team today to get started!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Customizable PTFE Three Neck Flasks for Advanced Chemical Applications
People Also Ask
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments



















