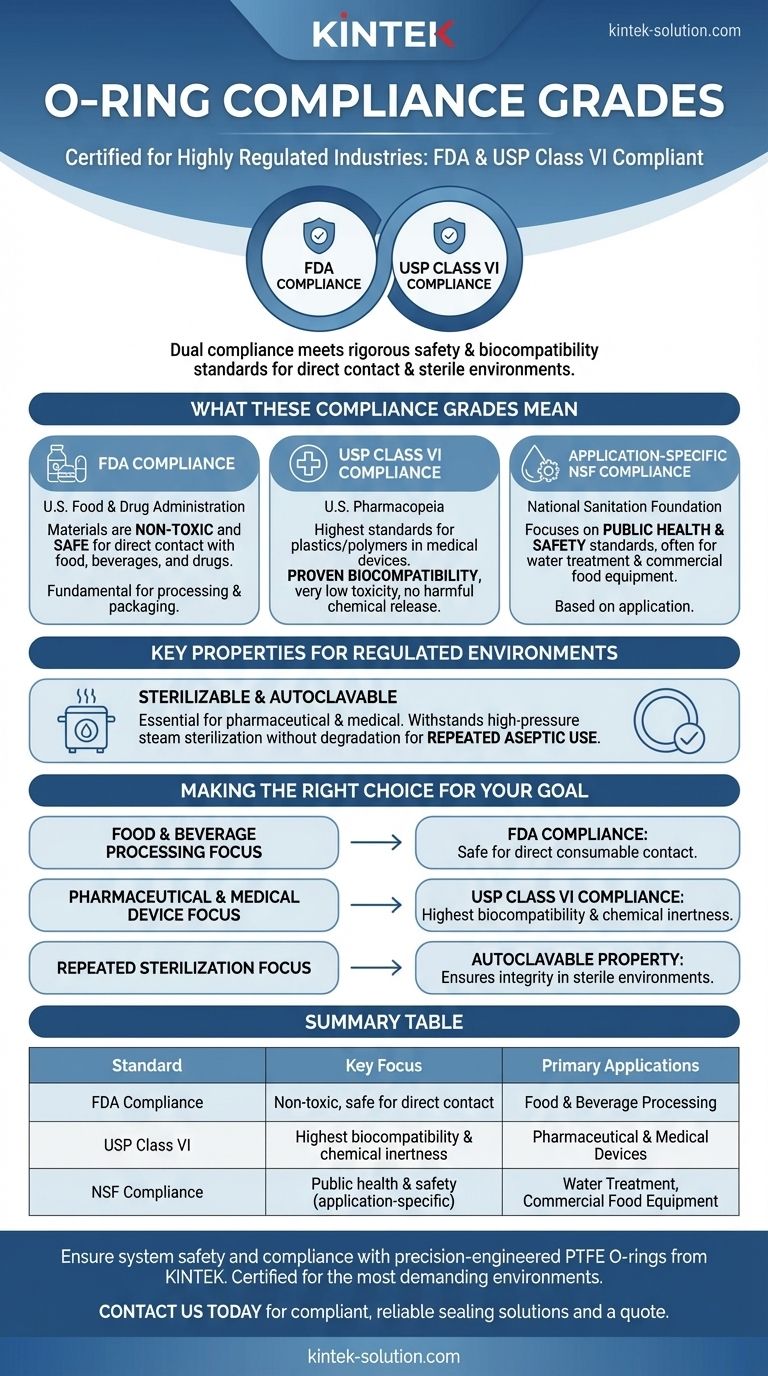
To be clear, these O-rings are certified for use in highly regulated industries. They are compliant with both FDA and USP Class VI standards, which are critical benchmarks for applications in the food, beverage, pharmaceutical, and medical device sectors.
The dual compliance with both FDA and USP Class VI signifies that these O-rings meet rigorous safety and biocompatibility standards, making them suitable for direct contact with consumables and for use in sterile medical environments.

What These Compliance Grades Mean
Understanding the specific standards an O-ring meets is essential for ensuring product safety, purity, and regulatory adherence in your application. Each certification addresses a distinct set of safety requirements.
FDA Compliance
The U.S. Food and Drug Administration (FDA) sets standards for materials that come into contact with food, beverages, and drugs.
FDA compliance ensures the O-ring's materials are considered non-toxic and safe for direct contact with products intended for human consumption. This is a fundamental requirement for any component used in food processing or packaging equipment.
USP Class VI Compliance
The U.S. Pharmacopeia (USP) sets the most stringent standards for plastics and polymers used in medical devices and containers.
USP Class VI is the highest and most rigorous of these standards. Materials must undergo a series of biological tests to prove a very low level of toxicity and high biocompatibility, ensuring they will not release harmful chemicals into the body or a drug product.
Application-Specific NSF Compliance
For certain use cases, NSF (National Sanitation Foundation) compliance is also available.
This certification focuses on public health and safety standards, often related to water treatment systems or commercial food equipment. Its availability depends on the specific application requirements.
Key Properties for Regulated Environments
Beyond material grades, these O-rings possess functional properties that are critical for maintaining sterile and pure operating conditions.
Sterilizable and Autoclavable
The ability to be sterilized is non-negotiable in pharmaceutical and medical applications.
These O-rings are also autoclavable, meaning they can withstand sterilization using high-pressure saturated steam without degrading. This allows for repeated use in processes where maintaining an aseptic, or sterile, environment is paramount.
Making the Right Choice for Your Goal
Selecting the correct component is a matter of matching its certifications to the demands of your industry and application.
- If your primary focus is food and beverage processing: FDA compliance is your key assurance that the material is safe for direct contact with consumables.
- If your primary focus is pharmaceutical or medical device manufacturing: USP Class VI compliance provides the highest level of confidence in the material's biocompatibility and chemical inertness.
- If your primary focus involves repeated sterilization cycles: The autoclavable property is critical for ensuring the O-ring maintains its integrity and performance in a sterile environment.
Ultimately, choosing components with these verified certifications is a foundational step in building a safe, reliable, and compliant system.
Summary Table:
| Standard | Key Focus | Primary Applications |
|---|---|---|
| FDA Compliance | Non-toxic, safe for direct contact | Food & Beverage Processing |
| USP Class VI | Highest biocompatibility & chemical inertness | Pharmaceutical & Medical Devices |
| NSF Compliance | Public health & safety (application-specific) | Water Treatment, Commercial Food Equipment |
Ensure your system's safety and compliance with precision-engineered PTFE O-rings from KINTEK.
We manufacture high-purity PTFE components, including seals and O-rings, that are certified for the most demanding environments in the semiconductor, medical, laboratory, and industrial sectors. Whether you need prototypes or high-volume orders, our custom fabrication ensures your components meet exact regulatory requirements.
Contact us today to discuss your application and receive a quote for compliant, reliable sealing solutions.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
People Also Ask
- What are the key advantages of PTFE? Unmatched Performance for Extreme Environments
- Why is PTFE rod suitable for automotive applications? Boost Vehicle Performance & Durability
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What are the main advantages of using PTFE parts in industrial applications? Unlock Unmatched Chemical Resistance and Reliability
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support



















