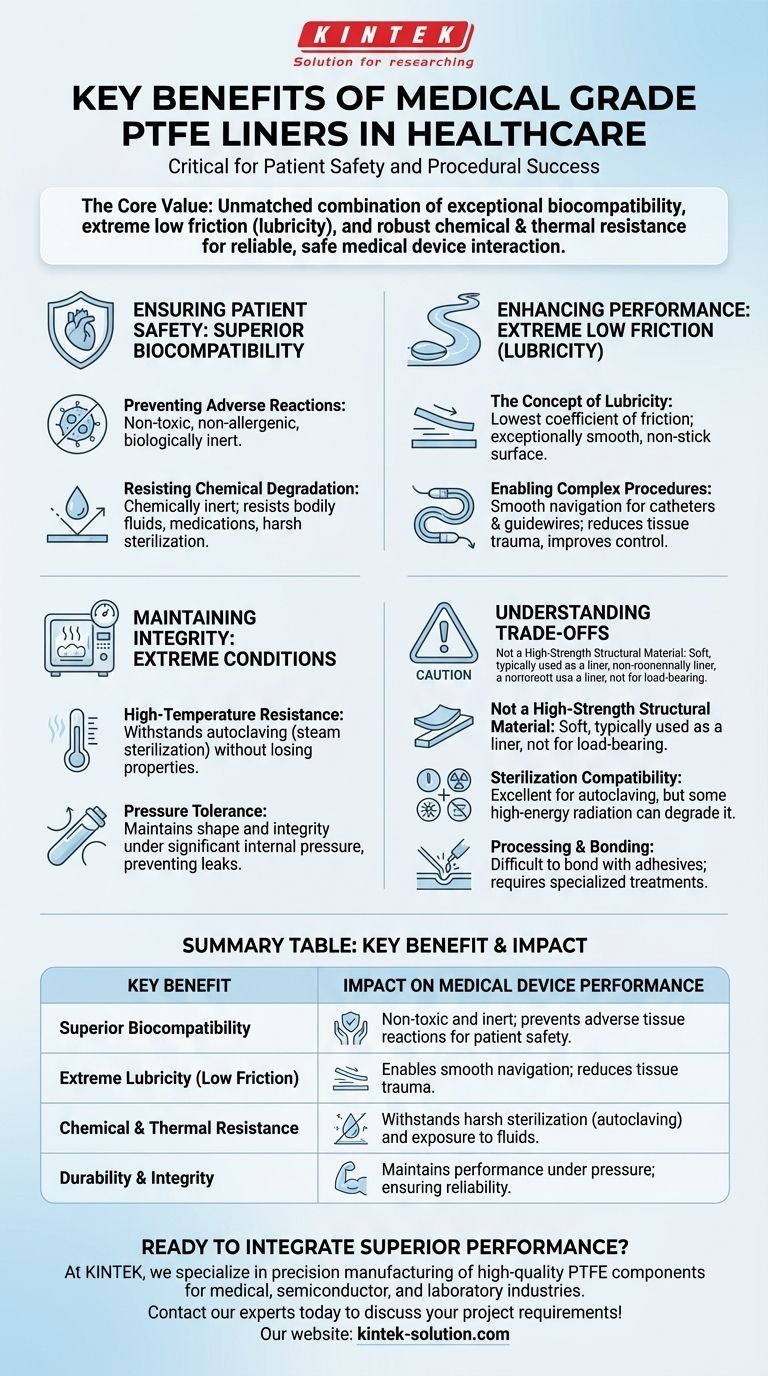
In medical device engineering, the choice of material is not just a technical detail—it is a critical factor for patient safety and procedural success. Medical Grade Polytetrafluoroethylene (PTFE) liners are chosen for their unique combination of exceptional biocompatibility, extreme low friction (lubricity), and robust chemical and thermal resistance. These properties ensure that devices interact safely with human tissue, perform reliably under harsh conditions, and enable complex medical procedures with greater precision.
The core value of Medical Grade PTFE lies in its dual ability to be both incredibly slick and biologically inert. This allows medical instruments to navigate the delicate internal pathways of the human body with minimal tissue trauma while ensuring the material itself will not cause an adverse reaction.
Ensuring Patient Safety with Superior Biocompatibility
The primary concern for any material used inside the body is its interaction with biological systems. Medical Grade PTFE excels in this area, providing a stable and safe interface.
Preventing Adverse Reactions
Biocompatibility means the material is non-toxic, non-allergenic, and does not trigger an immune or inflammatory response from the body. PTFE is one of the most biologically inert polymers known, making it a trusted choice for implants and devices with direct, long-term tissue contact.
Resisting Chemical Degradation
PTFE is almost completely chemically inert. It will not break down or corrode when exposed to bodily fluids, aggressive medications, or harsh sterilization agents. This ensures the device's structural integrity and prevents harmful substances from leaching into the patient's system.
Enhancing Device Performance Through Extreme Low Friction
The surface properties of PTFE are central to its function in many medical devices, particularly those used for diagnostics and interventions.
The Concept of Lubricity
PTFE has one of the lowest coefficients of friction of any solid material, a property often referred to as lubricity. This creates an exceptionally smooth, non-stick surface that allows other components or instruments to glide over it with minimal force.
Enabling Complex Procedures
This extreme lubricity is critical for devices like catheters and guidewires. It allows physicians to navigate them through delicate and tortuous blood vessels or other anatomical pathways smoothly and precisely, reducing tissue trauma and improving operator control.
Maintaining Integrity in Extreme Conditions
Medical devices must function flawlessly and withstand rigorous pre-use processing, such as sterilization. PTFE's resilience ensures reliability from manufacturing to the operating room.
High-Temperature Resistance
Medical instruments must be sterilized to prevent infection. PTFE liners can easily withstand the high temperatures and pressures of autoclaving (steam sterilization), one of the most common and effective methods, without losing their physical properties.
Pressure Tolerance
In applications like catheter-based delivery systems, liners must withstand significant internal pressure. PTFE's durability ensures that the device maintains its shape and integrity, preventing leaks or failures during critical procedures.
Understanding the Trade-offs and Considerations
While its benefits are significant, it's crucial to understand the limitations of PTFE to use it effectively. Objectivity requires acknowledging where other materials might be more suitable.
Not a High-Strength Structural Material
Pure PTFE is a relatively soft material. While durable and tough, it is not designed for load-bearing structural components. This is why it is most often used as a liner or coating, leveraging its surface properties while relying on another material for rigidity.
Sterilization Method Compatibility
While excellent for autoclaving, certain sterilization methods like high-energy gamma radiation can degrade PTFE's mechanical properties over time. Engineers must match the material to the required sterilization protocol for the device.
Processing and Bonding
The same chemical inertness that makes PTFE biocompatible also makes it very difficult to bond to other materials using conventional adhesives. Specialized surface treatments are often required, which can add complexity and cost to the manufacturing process.
Making the Right Choice for Your Application
Selecting the right material is about aligning its properties with your primary design goal. PTFE liners offer a clear advantage in several key areas.
- If your primary focus is patient safety and direct tissue contact: Prioritize PTFE for its unmatched biocompatibility and chemical inertness to eliminate the risk of adverse biological reactions.
- If your primary focus is maneuverability in delivery systems: Leverage PTFE's extreme lubricity to design catheters and guidewires that navigate complex anatomy with minimal friction and maximum control.
- If your primary focus is device longevity and sterilizability: Choose PTFE for its proven ability to withstand repeated high-temperature steam sterilization cycles without degrading.
Ultimately, specifying a Medical Grade PTFE liner is a strategic decision to build reliability, performance, and patient safety into the core of your medical device.
Summary Table:
| Key Benefit | Impact on Medical Device Performance |
|---|---|
| Superior Biocompatibility | Non-toxic and inert, prevents adverse tissue reactions for patient safety. |
| Extreme Lubricity (Low Friction) | Enables smooth navigation of catheters and guidewires, reducing tissue trauma. |
| Chemical & Thermal Resistance | Withstands harsh sterilization (autoclaving) and exposure to bodily fluids/medications. |
| Durability & Integrity | Maintains performance under pressure, ensuring device reliability during procedures. |
Ready to integrate the superior performance of Medical Grade PTFE into your medical device?
At KINTEK, we specialize in the precision manufacturing of high-quality PTFE components—including seals, liners, and custom labware—for the medical, semiconductor, and laboratory industries. Our expertise ensures your devices benefit from unmatched biocompatibility, lubricity, and durability.
We offer custom fabrication from prototypes to high-volume orders, tailored to your exact specifications. Let us help you enhance patient safety and device reliability.
Contact our experts today to discuss your project requirements!
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What are the future considerations for machining Teflon? Mastering Material Challenges with Smart Tech
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers



















