To be direct, PTFE and Teflon possess exceptional chemical resistance, making them two of the most chemically inert materials used in industry. For practical purposes, Teflon is simply a brand name for the polymer Polytetrafluoroethylene (PTFE). It is virtually immune to attack by the vast majority of chemicals, including aggressive acids, bases, solvents, and hydrocarbons.
The core principle to understand is that PTFE's near-universal chemical inertness comes from its extremely stable molecular structure. However, this stability can be overcome by a very short list of highly reactive substances, primarily molten alkali metals and powerful fluorinating agents under specific conditions.

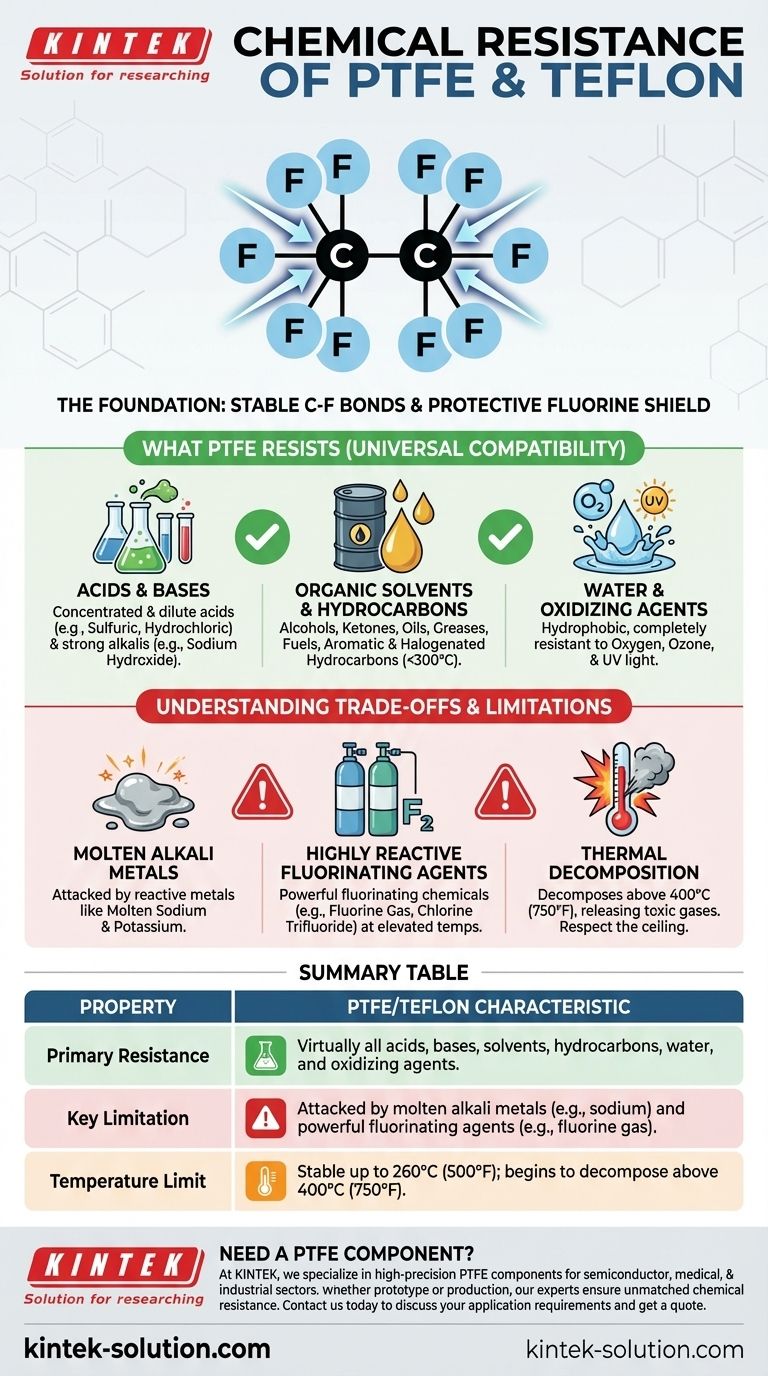
The Foundation of PTFE's Chemical Inertness
The remarkable resistance of PTFE is not an accident; it is a direct result of its molecular architecture. Understanding this foundation is key to trusting its capabilities.
The Carbon-Fluorine Bond
At its heart, PTFE consists of a long chain of carbon atoms, each completely shielded by fluorine atoms. The bond between carbon and fluorine (C-F) is one of the strongest single bonds known in organic chemistry.
This powerful bond requires a tremendous amount of energy to break, which is why most chemicals simply cannot react with it.
A Protective Fluorine Shield
The fluorine atoms are larger than the carbon atoms they surround. They effectively form a dense, non-reactive sheath around the vulnerable carbon backbone, protecting it from chemical attack.
This structure also results in a material that is hydrophobic (repels water) and oleophobic (repels oil), preventing substances from wetting its surface and initiating a reaction.
What PTFE Resists (Universal Compatibility)
Due to its stable structure, PTFE is the material of choice for applications in chemically aggressive environments. It is effectively a universal solution for most scenarios.
Acids and Bases
PTFE is highly resistant to both concentrated and dilute acids, as well as strong alkalis (bases). This includes substances like sulfuric acid, hydrochloric acid, and sodium hydroxide.
Organic Solvents and Hydrocarbons
It does not dissolve in any known solvent at temperatures below 300°C. It stands up perfectly to alcohols, ketones, oils, greases, fuels, and both aromatic and halogenated hydrocarbons.
Water and Oxidizing Agents
PTFE does not absorb water and is completely resistant to damage from oxygen, ozone, and UV light. Its properties do not degrade with exposure to the elements.
Understanding the Trade-offs and Limitations
No material is perfect. While PTFE's list of incompatibilities is extremely short, they are critical to know to prevent catastrophic failure in specialized applications.
The Primary Exceptions: Molten Alkali Metals
Highly reactive alkali metals like molten sodium and potassium are energetic enough to strip the fluorine atoms from the polymer backbone, causing the material to degrade.
Highly Reactive Fluorinating Agents
Certain powerful fluorinating chemicals can attack PTFE, especially at elevated temperatures and pressures. These are not common industrial chemicals and include substances like fluorine gas (F₂), chlorine trifluoride (ClF₃), and xenon difluoride (XeF₂).
Thermal Decomposition
While not a chemical reaction in the typical sense, it's a critical limitation. Above 400°C (750°F), PTFE begins to decompose, releasing toxic and corrosive fluorocarbon gases. This temperature ceiling must be respected in any high-heat application.
Making the Right Choice for Your Application
To apply this knowledge, consider the specific chemical and thermal demands of your operating environment.
- If your primary focus is general industrial or laboratory use: PTFE is almost certainly one of the safest and most reliable material choices available for gaskets, seals, linings, and tubing.
- If your primary focus is a high-temperature environment: You must ensure that operating temperatures remain well below the 400°C decomposition threshold to avoid material failure and hazardous off-gassing.
- If you are working with molten alkali metals or aggressive fluorinating agents: You fall into the rare category where PTFE is unsuitable and an alternative material must be sourced.
Ultimately, understanding both PTFE's remarkable strengths and its precise limitations is the mark of sound engineering.
Summary Table:
| Property | PTFE/Teflon Characteristic |
|---|---|
| Primary Resistance | Virtually all acids, bases, solvents, hydrocarbons, water, and oxidizing agents. |
| Key Limitation | Attacked by molten alkali metals (e.g., sodium) and powerful fluorinating agents (e.g., fluorine gas). |
| Temperature Limit | Stable up to 260°C (500°F); begins to decompose above 400°C (750°F). |
Need a PTFE component that can withstand your most aggressive chemical processes?
At KINTEK, we specialize in manufacturing high-precision PTFE components—including seals, liners, and labware—for the semiconductor, medical, laboratory, and industrial sectors. Whether you require a custom prototype or a high-volume production run, our expertise ensures your components deliver unmatched chemical resistance and reliability.
Contact our experts today to discuss your specific application requirements and get a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- What industries use PTFE machined parts and for what applications? Critical Components for Demanding Environments
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs



















