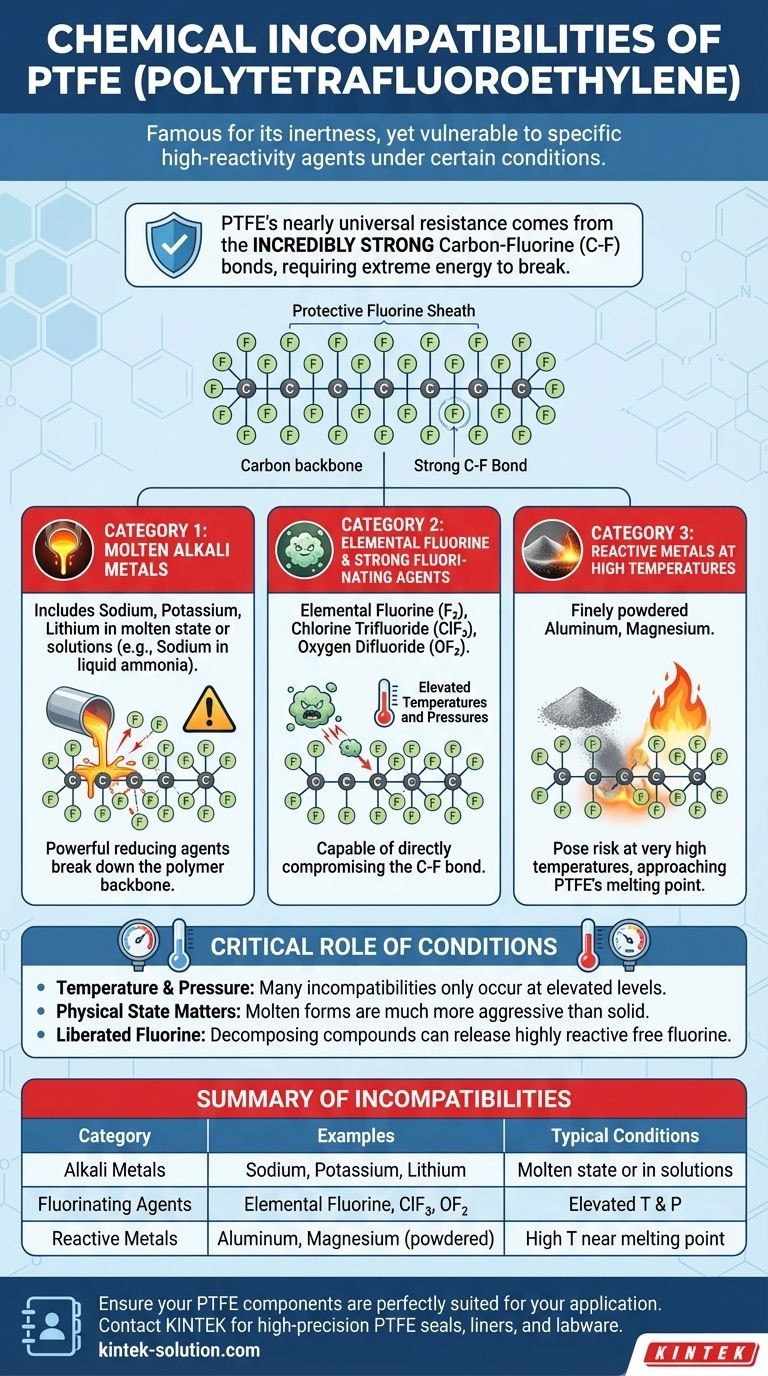
Yes, while PTFE is famously inert, it is not compatible with a very specific and limited list of highly reactive chemicals under certain conditions. These exceptions primarily include molten alkali metals, elemental fluorine and other aggressive fluorinating agents, and certain reactive metals at very high temperatures. For the vast majority of acids, bases, solvents, and organic compounds, PTFE remains one of the most resistant polymers available.
PTFE’s near-universal chemical resistance stems from its incredibly strong carbon-fluorine bonds. The few substances that can attack it are those powerful enough to break these bonds, a reaction that often requires extreme conditions like high heat or pressure.

Why PTFE is So Chemically Resistant
Before examining the exceptions, it's important to understand why PTFE (polytetrafluoroethylene) has its reputation for being almost completely inert. The material's resilience is not magic; it's a direct result of its molecular structure.
The Strength of the Carbon-Fluorine Bond
At its core, PTFE consists of a long chain of carbon atoms, where each carbon is bonded to two fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest single bonds in organic chemistry.
This structure effectively creates a protective sheath of fluorine atoms around the carbon backbone, shielding it from chemical attack. Breaking these bonds requires a tremendous amount of energy, which most chemicals simply cannot deliver.
A Non-Polar, Low-Energy Surface
The fluorine sheath also creates a very dense, non-polar surface with low surface energy. This is why PTFE is hydrophobic (repels water) and oleophobic (repels oil), and it contributes to its non-stick properties. Most chemical reactants are unable to "wet" or effectively make contact with the surface to initiate a reaction.
The Specific Chemical Incompatibilities
Despite its robustness, a few substances are reactive enough to overcome PTFE's defenses. These reactions are typically aggressive and often require specific environmental conditions.
Category 1: Alkali Metals
PTFE is vulnerable to attack from molten alkali metals such as sodium, potassium, and lithium. It can also be affected by these metals when they are in certain solutions, like sodium in liquid ammonia.
These metals are powerful reducing agents that can physically strip fluorine atoms from the polymer backbone, causing a breakdown of the material's structure.
Category 2: Elemental Fluorine and Strong Fluorinating Agents
It may seem counterintuitive, but PTFE can be attacked by elemental fluorine (especially turbulent liquid or gaseous fluorine) and other extremely aggressive fluorinating compounds.
Chemicals like chlorine trifluoride (ClF₃) and oxygen difluoride (OF₂) can attack PTFE, particularly at elevated temperatures and pressures. These substances are among the few capable of directly compromising the C-F bond.
Category 3: Other Reactive Metals at High Temperatures
Beyond alkali metals, other highly reactive metals can pose a risk, but typically only at very high temperatures.
Finely powdered aluminum and magnesium, for example, can react with PTFE at temperatures approaching its melting point. This is not a common scenario but is a critical consideration in specialized high-temperature applications.
Understanding the Trade-offs: The Critical Role of Conditions
Knowing the list of chemicals is only half the story. Chemical compatibility is not always a simple yes or no; it is heavily dependent on the operating environment.
Temperature and Pressure Are Key
Many of the incompatibilities noted above only occur at elevated temperatures and/or pressures. At room temperature and standard pressure, PTFE might show no reaction even to some of the listed chemicals in a less active state. Always evaluate compatibility based on your specific process conditions.
Physical State Matters
The physical state of the chemical is crucial. For instance, a solid block of sodium metal at room temperature will not react with PTFE. However, molten sodium is extremely aggressive and will readily attack it.
Beware of Liberated Fluorine
Some fluorine-containing compounds, while not inherently reactive with PTFE, can decompose at high temperatures and liberate free fluorine radicals. This free fluorine is highly reactive and can then attack the PTFE structure, causing degradation.
Making the Right Choice for Your Application
Understanding these nuances allows you to select materials with confidence and ensure the safety and integrity of your process.
- If your primary focus is general lab or industrial use with acids, bases, or solvents: PTFE remains one of the most reliable and inert materials available for these common applications.
- If your primary focus is high-temperature processes involving reactive metals: You must verify compatibility, as molten alkali metals or even powdered aluminum and magnesium can cause degradation.
- If your primary focus involves handling elemental fluorine or aggressive fluorinating agents: PTFE is not a suitable material, especially under heat and pressure, and you must seek specialized alternatives.
By understanding these specific limitations, you can confidently deploy PTFE where it excels and avoid risks in highly reactive environments.
Summary Table:
| Incompatible Category | Examples | Typical Conditions |
|---|---|---|
| Alkali Metals | Sodium, Potassium, Lithium | Molten state or in solutions (e.g., sodium in liquid ammonia) |
| Fluorinating Agents | Elemental Fluorine, Chlorine Trifluoride (ClF₃) | Elevated temperatures and pressures |
| Reactive Metals | Aluminum, Magnesium (finely powdered) | High temperatures approaching PTFE's melting point |
Ensure your PTFE components are perfectly suited for your application. At KINTEK, we specialize in manufacturing high-precision PTFE seals, liners, and labware for the semiconductor, medical, laboratory, and industrial sectors. Whether you need custom prototypes or high-volume orders, our expertise ensures your components withstand your specific chemical and thermal conditions. Contact us today to discuss your requirements and benefit from our tailored fabrication solutions!
Visual Guide
Related Products
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What is expanded PTFE (ePTFE) and how is it made? Unlock the Power of a Microporous Wonder Material
- What are the general chemical resistance properties of PTFE and Teflon? Unmatched Inertness for Demanding Applications
- What makes PTFE plastic uniquely versatile across industries? The 4 Key Properties Explained
- What are some applications of PTFE due to its properties? Discover Its Versatility in Demanding Industries
- What are filled PTFE materials and what are their benefits? Enhance Performance for Demanding Applications
- Which industries commonly use Teflon and why? Its Unique Properties Solve Critical Engineering Challenges
- What is PTFE commonly known as and what type of material is it? A Guide to High-Performance PTFE Properties
- How does PTFE perform in chemical-heavy environments? Unmatched Chemical Resistance for Demanding Applications



















